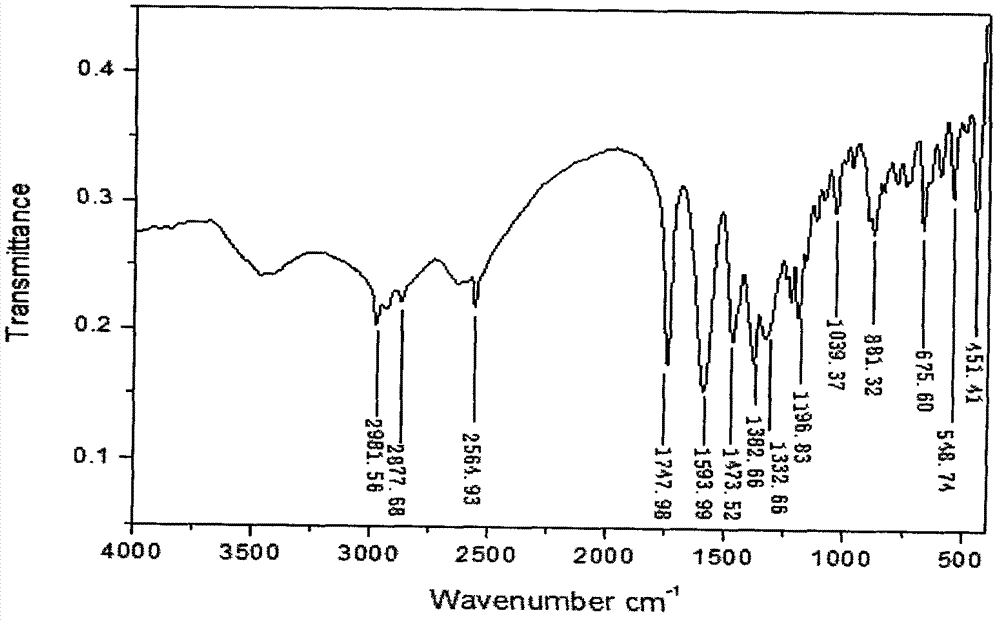
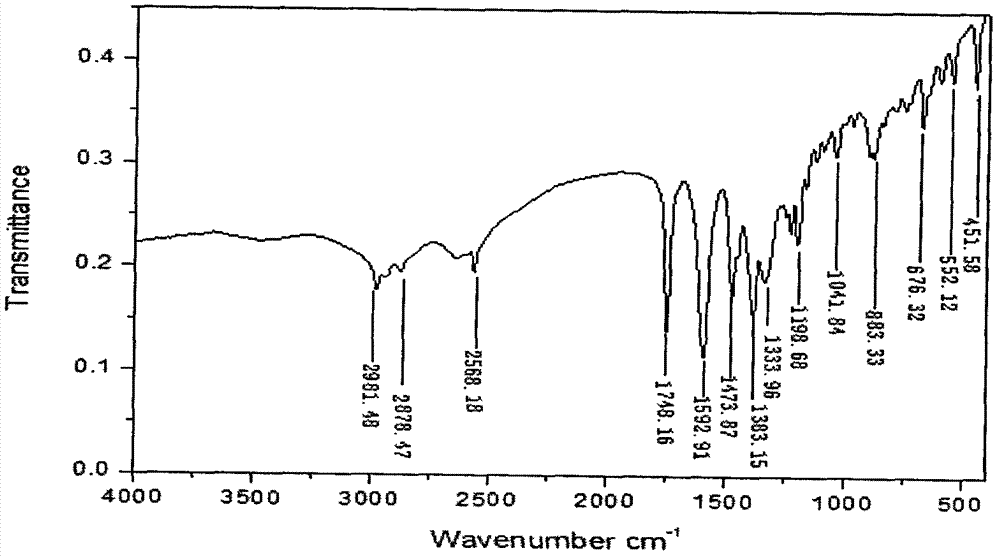
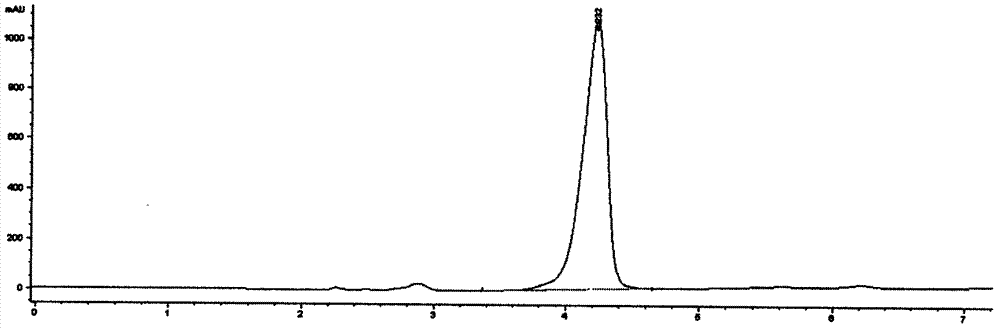
Synthetic method of 1-(3-mercapto-2-D-methyl propionyl)-L-proline
The technology of a methacryl group and a synthetic method is applied in the field of preparation of 1--L-proline, which can solve the problems of many side reactions, long reaction time, difficulty in control, etc., achieve mild reaction conditions, short reaction time, Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Adopt a 2L three-necked flask equipped with a stirring blade and a thermometer, and the reaction temperature is controlled by an oil bath, and operates as follows:
[0030] (1) Add 43.1g (0.51mol) sodium bicarbonate solid in a three-necked flask, add 392mL of water and stir to dissolve (the mass concentration of sodium bicarbonate solution is 11%), then add 135g (0.51mol) of 1-(3 -Bromo-2-D-methylpropionyl)pyrrolidine-2-carboxylic acid, reacted at room temperature for 5 minutes, and then heated to 70°C;
[0031] (2) Add 139g (0.56mol) sodium thiosulfate pentahydrate (NaS 2 o 3 .5H 2O) solid, reacted at 70° C. for 2 hours, then cooled to room temperature;
[0032] (3) 223 g (0.29 mol) of sodium sulfide solution with a mass concentration of 10% was added to the above reaction solution, and reacted at room temperature for 25 minutes;
[0033] (4) acidify the above reaction solution with hydrochloric acid to pH=2, react at room temperature for 25 minutes, and filter to ...
Embodiment 2
[0038] Reaction device, technological process are with embodiment 1. Other steps processing conditions are with embodiment 1. Only the process conditions of step (1) are adjusted to: add 43.1g (0.51mol) of sodium bicarbonate solid in a three-necked flask, add water 287mL and stir to dissolve (the mass concentration of sodium bicarbonate solution is 20%). Finally, 119.8 g (0.55 mol) of disulfide solids of 1-(3-mercapto-2-D-methylpropionyl)-L-proline were obtained, yield: 108%; 1-(3-mercapto-2 -D-methylpropionyl)-L-proline solid 88.3g (0.41mol), yield: 74%, content: 97.3%, total yield: 80%.
Embodiment 3
[0040] Reaction device, technological process are with embodiment 1. Other steps processing conditions are with embodiment 1. Only the process condition of step (2) is adjusted to: react at 80° C. for 1 hour. Finally, 120.0 g (0.55 mol) of disulfide solids of 1-(3-mercapto-2-D-methylpropionyl)-L-proline were obtained, yield: 108%; 1-(3-mercapto-2 -D-methylpropionyl)-L-proline solid 89.5g (0.41mol), yield: 75%, content: 97.5%, total yield: 81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com