K<+>/H<+>antiporter protein, its coding gene, and applications of protein and coding gene
A protein and gene technology, applied in the field of coding genes of potassium hydrogen pump protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
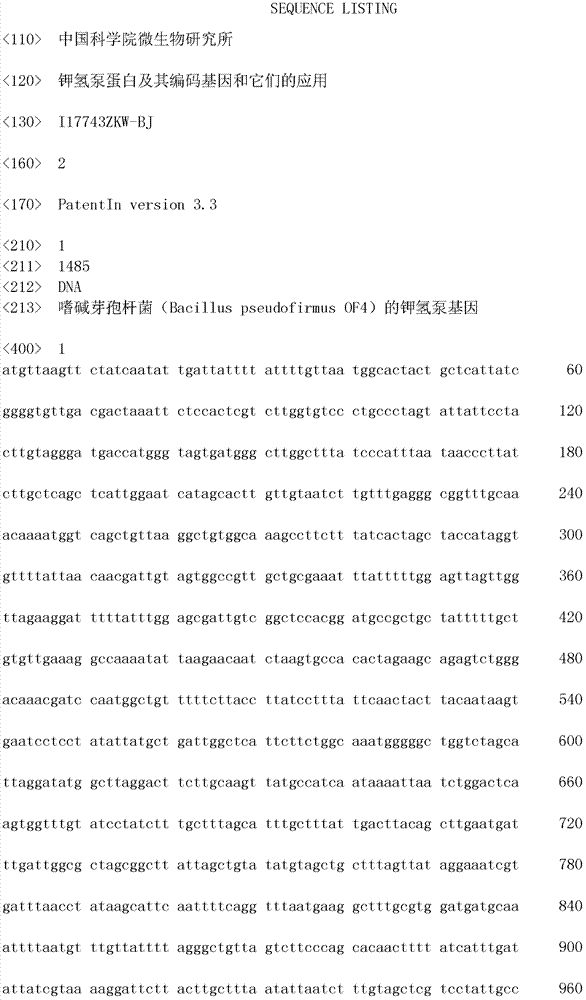
[0021] Cloning of Nucleotide Sequence Encoding Potassium Hydrogen Pump
[0022] (1) Extraction and purification of total DNA of Bacillus pseudofirmus OF4
[0023] Take 20 grams of fresh wet bacteria of Bacillus pseudofirmus OF4, suspend in 10 ml of 50 mmol / L Tris buffer solution (pH 8.0), add a small amount of lysozyme and 8 ml of 0.25 mmol / L ethylene glycol Eminetetraacetic acid (EDTA) (pH 8.0), mix well and place at 37°C for 20 minutes, then add 2 ml of 10% sodium dodecyl sulfate (SDS), place at 55°C for 5 minutes, add equal volumes of phenol and chloroform respectively Each extraction was performed once, and the supernatant solution of the last extraction was taken, and 2 times the volume of ethanol was added to precipitate DNA. After the DNA recovered from the precipitation was washed successively with 70 volume % ethanol solution and absolute ethanol, the obtained DNA was dissolved in 0.5 ml TE buffer solution (pH 8.0, 10 mmol / L Tris, 1 mmol / L EDTA), and 10 3 microliter...
Embodiment 2
[0026] Example 2 Verification of the gene function encoding the potassium-hydrogen pump
[0027] (1) Construction of recombinant cloning vector pUC18-BpOF4-cha
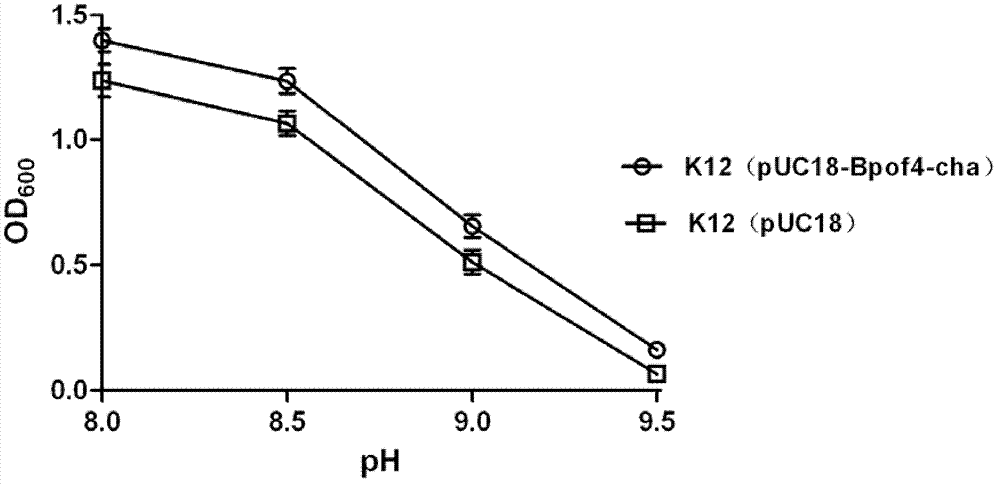
[0028] The PCR product obtained above was purified using Cycle-pure Kit. The purified PCR product was ligated with pUC18 using Fast clone Kit, and the constructed recombinant vector pUC18-BpOF4-cha was introduced into Escherichia coli K12BW25113 by chemical transformation. The positive clone Escherichia coli K12 (pUC18-BpOF4-cha) containing the recombinant vector pUC18-BpOF4-cha was obtained by screening on the LB plate containing 100 μg / ml ampicillin and PCR verification, and the amplification of the pUC18-BpOF4-cha insertion was confirmed by sequencing. The augmented sequence is completely consistent with the nucleotide sequence of the potassium hydrogen pump.
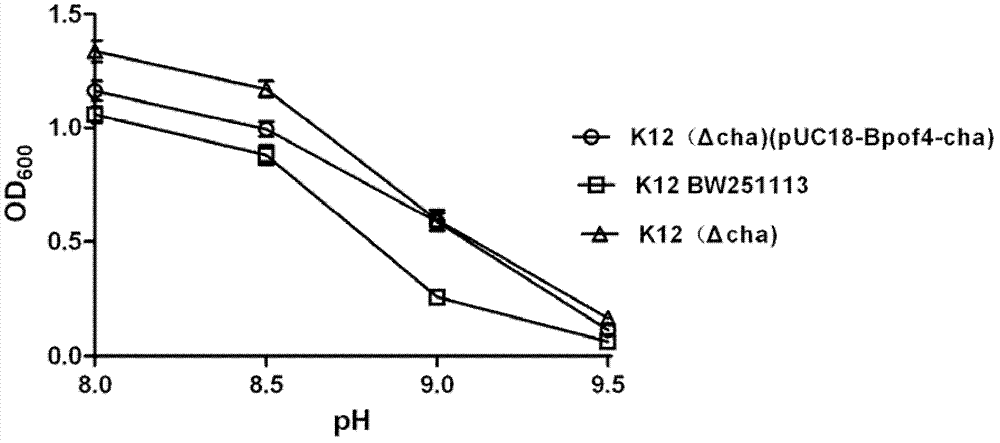
[0029] (2) Construction of Escherichia coli K12BW25113 potassium hydrogen pump deletion body
[0030]By designing primers containing the DNA fragments of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com