Biological thiol fluorescent probe as well as preparation method and application thereof
A technology of fluorescent probes and mercapto groups, which is applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, and luminescent materials. Improving the signal-to-noise ratio of imaging and the effect of high fluorescence stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
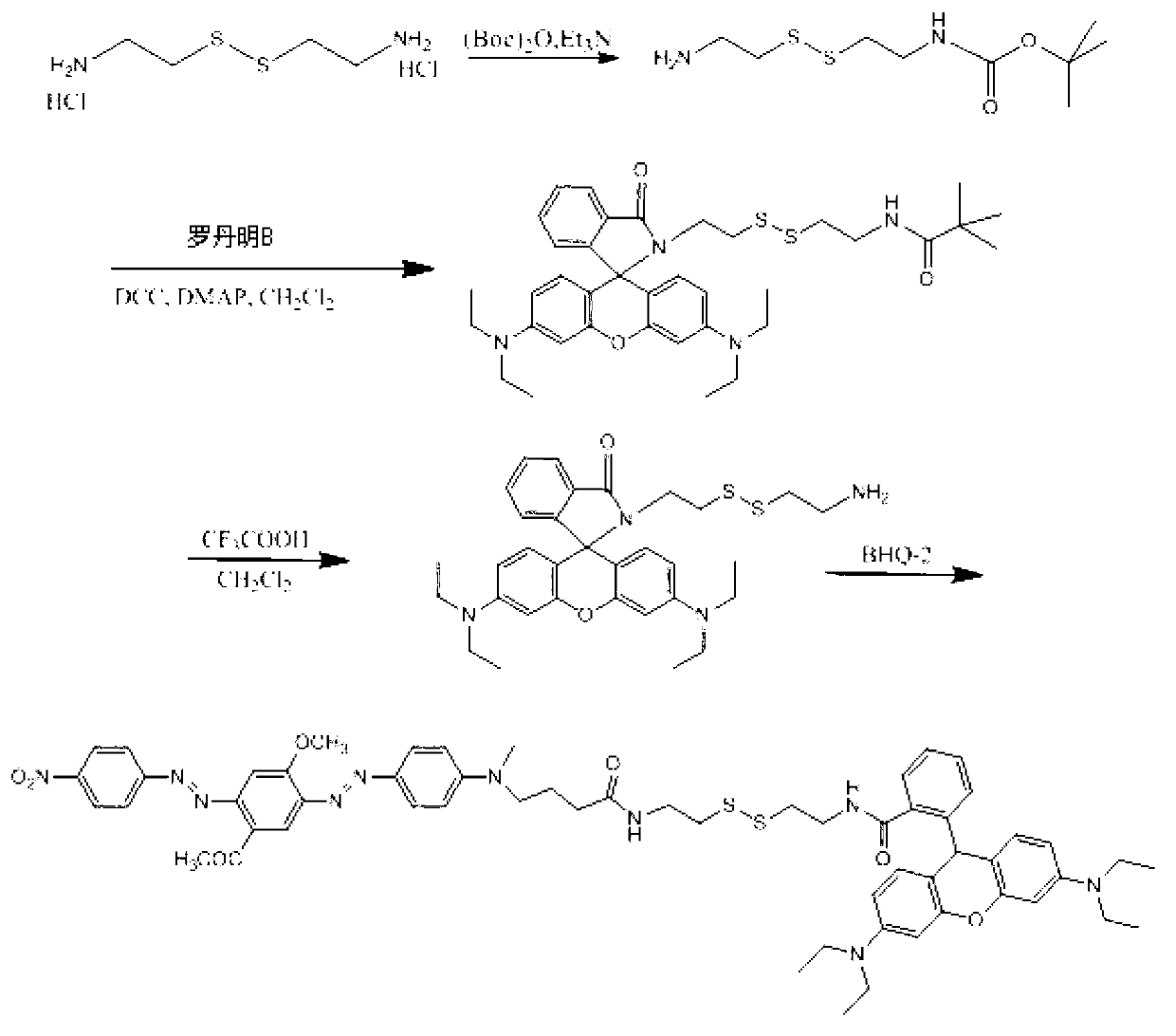
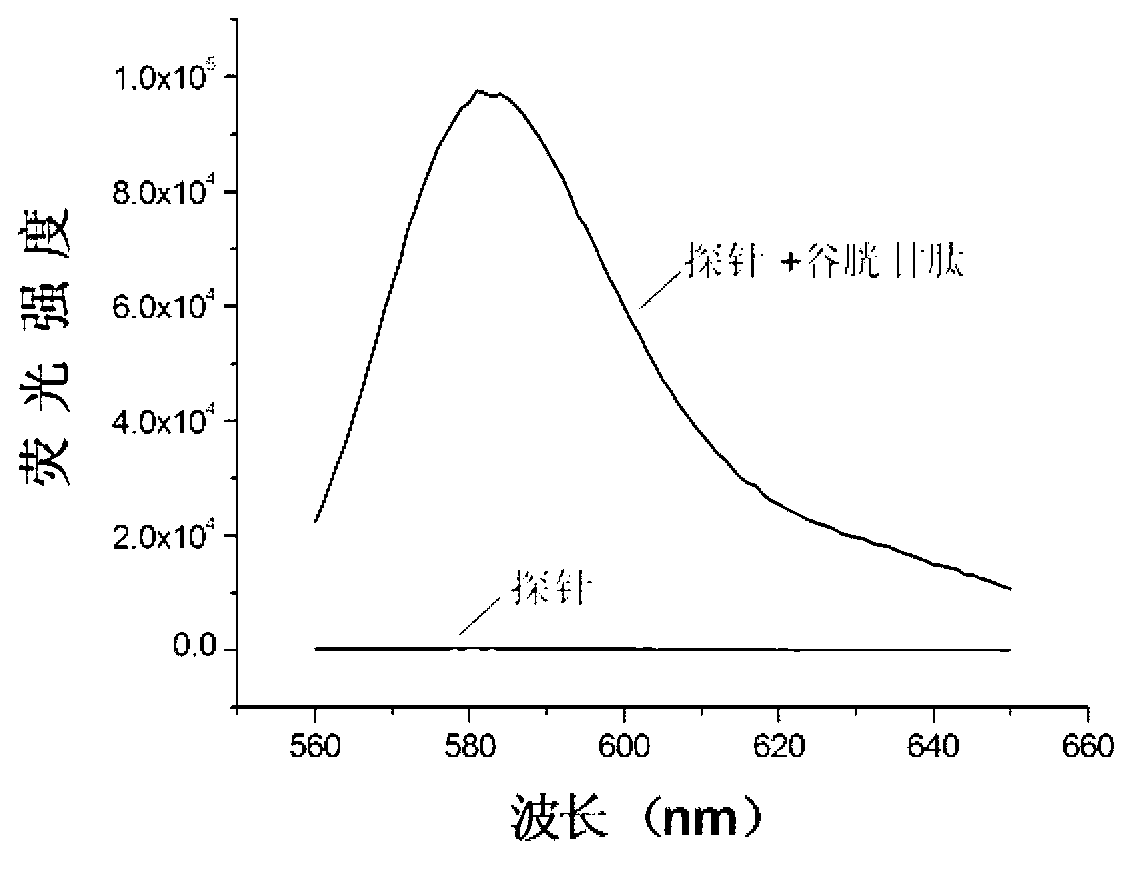
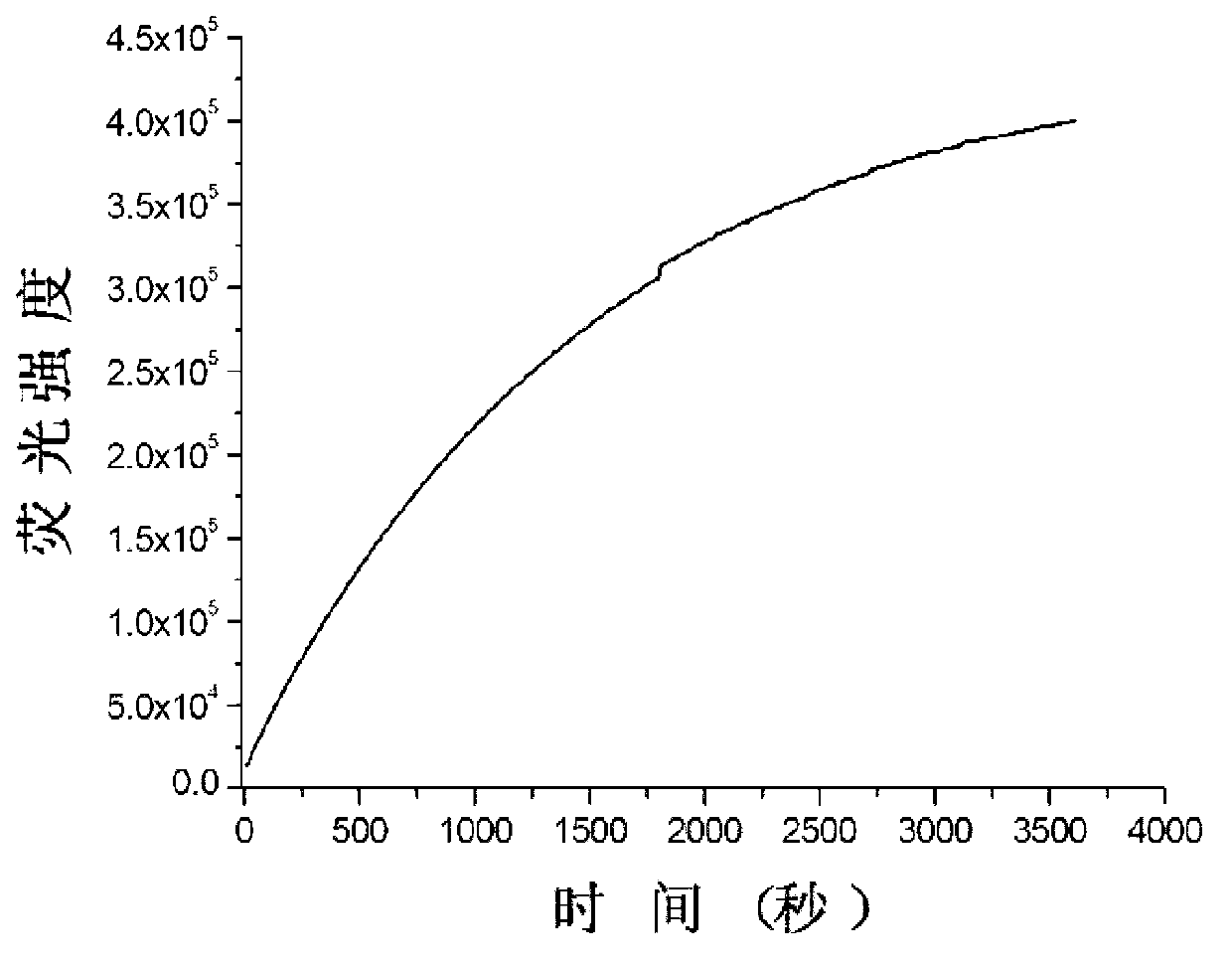
[0056] The biosulfhydryl fluorescent probe shown in formula II was synthesized by using the compound shown in formula III as the disulfide bond skeleton, Rhodamine B (Rhodamine B) biofluorescent group as the donor, and the quencher group of BHQ-2 as the acceptor.
[0057]
[0058] Synthetic route such as figure 1 As shown, the specific steps are as follows:
[0059] (1) Dissolve 2.24g of cystamine dihydrochloride in 25mL of methanol, then add 4.18mL of triethylamine to obtain the first mixed solution; dissolve 2.18g of di-tert-butyl dicarbonate in methanol, , use a pressure dropping funnel to drop the methanol solution dissolved in di-tert-butyl dicarbonate into the first mixed solution, and wait for 50 minutes until the addition is completed, and react for 5 hours to obtain semi-blocked cystamine; and perform purification.
[0060] (2) Dissolve 252 mg of semi-blocked cystamine obtained in step (1) with 20 mL of dichloromethane; add 479 mg of Rhodamine B, 226 mg of DCC, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Excitation wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com