1,8-dinitro-3,6-naphthalene disulfonate hydrogenation reduction method
A technology of naphthalene disulfonate and naphthalene disulfonic acid, applied in the preparation of sulfonic acid, organic chemistry, etc., can solve the problems of high chroma, high COD, etc., and achieve the effect of high economic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
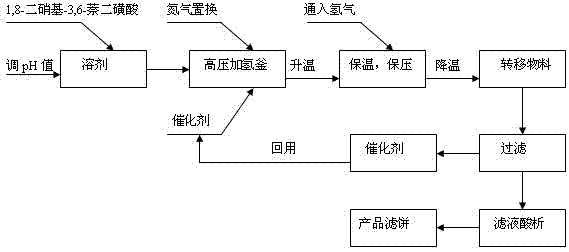
[0016] In 60g of methanol solution, add 40g of 1,8-dinitro-3,6-naphthalene disulfonic acid, and at the same time adjust the pH to 7-10 with ammonia water to convert into ammonium sulfonate, after stabilization, transfer to high-pressure hydrogenation Add 0.1g of ruthenium-silica gel supported catalyst to the kettle, seal and maintain the pressure, replace with nitrogen for 3 times, start to raise the temperature to 70°C, when the temperature is up, start to feed hydrogen, keep the pressure at 1.0MPa, keep the temperature for 5 hours, and turn on the cooling water, cooled down to normal temperature, and after pressure relief, the catalyst was removed by filtration to obtain the acid analysis of the filtrate. Filter and dry the filter cake. The yield of the target main product was 36.25g, the yield was 97.66%, and the purity of the target main product was 98.76%.
Embodiment example 2
[0018] In 80g of aqueous solution, add 20g of 1,8-dinitro-3,6-naphthalene disulfonic acid, and then use 30% liquid caustic soda to adjust the pH value to 7-10 and convert it into sodium sulfonate. Other operations are the same as in the examples 1. Using this embodiment, the yield of the target main product was 18.24 g, the yield was 98.27%, and the purity of the target main product was 99.03%.
Embodiment example 3
[0020] Change catalyst into palladium carbon catalyst 0.02g in case 1, all the other are the same as case 1. Finally, the yield of the target main product was 36.12g, the yield was 97.32%, and the purity of the target main product was 98.47%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com