Preparation method of 7-bromo-4-hydroxy-3-quinoline carboxylic acid
A quinoline carboxylic acid and hydroxyl technology, which is applied in the field of preparation of 7-bromo-4-hydroxy-3-quinoline carboxylic acid, can solve the problems of low product purity, troublesome post-processing, low product yield and the like, and achieves Overcome the effects of many by-products, easy separation and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
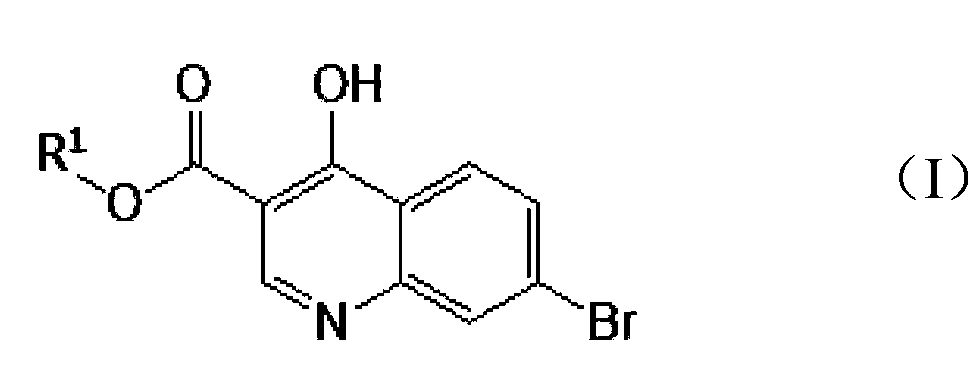
[0032] The preparation method of 7-bromo-4-hydroxyl-3-quinoline carboxylic acid provided by the invention comprises:
[0033] 7-bromo-4-hydroxyl-3-quinoline carboxylic acid (C 1~3 ) Alkyl ester
[0034]
[0035] Among them, R 1 It is methyl, ethyl, n-propyl or isopropyl, and an organic solvent such as absolute ethanol or absolute methanol and a hydrochloric acid aqueous solution with a concentration of 1-2mol / L are added to the reaction vessel, wherein the amount of the solvent is based on each gram 7-bromo-4-hydroxyl-3-quinolinecarboxylic acid (C 1~3 ) Alkyl ester needs to add in the ratio of 25mL of solvent, 7-bromo-4-hydroxyl-3-quinolinecarboxylic acid (C 1~3 ) The molar ratio of alkyl ester to hydrochloric acid is 1:3-5, stirring and heating to reflux for 0.5-2 hours, preferably 1-1.5 hours, a large amount of solid products are precipitated, after the reaction is completed, filter, and the filtrate is concentrated until solids are precipitated , combined filter cake...
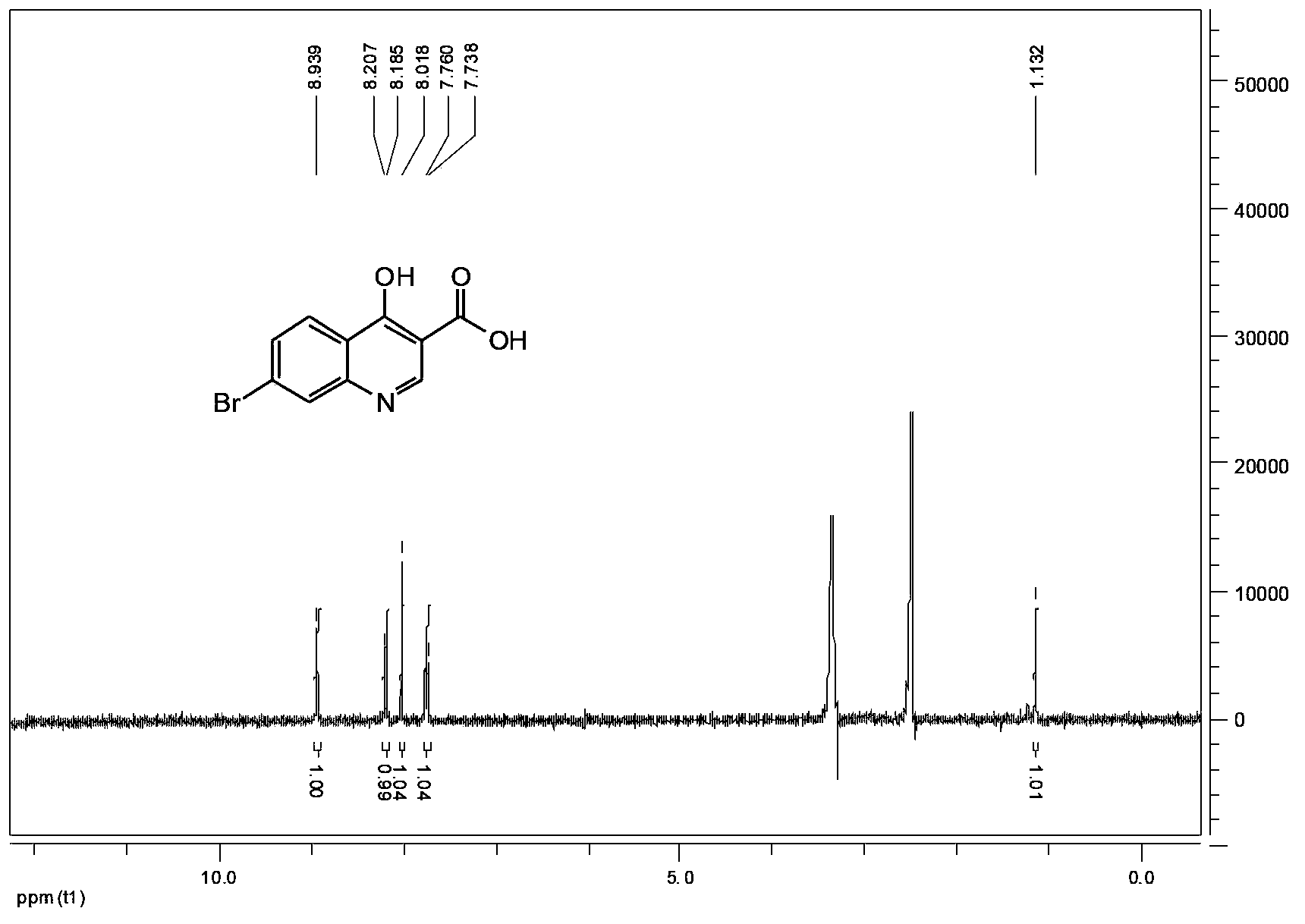
Embodiment 1
[0053] Preparation of 7-bromo-4-hydroxy-3-quinolinecarboxylic acid
[0054] Add 28.7g of analytically pure ethyl 7-bromo-4-hydroxy-3-quinolinecarboxylate (commercially available), 720mL of anhydrous methanol and 147mL of 1mol / L hydrochloric acid aqueous solution into the reaction flask, stir and heat to reflux for 1.5h, There is a large amount of solid product to separate out, after the reaction is completed, filter, the filtrate is concentrated until the solid is separated out, the combined filter cake is washed 3 times with methanol, and after drying, 22.7g of white solid 7-bromo-4-hydroxyl-3-quinolinecarboxylic acid is obtained , The calculated yield was 87.5%, and the purity of 7-bromo-4-hydroxyl-3-quinolinecarboxylic acid obtained by Agilent1100 liquid chromatography was 99.0%.
Embodiment 2
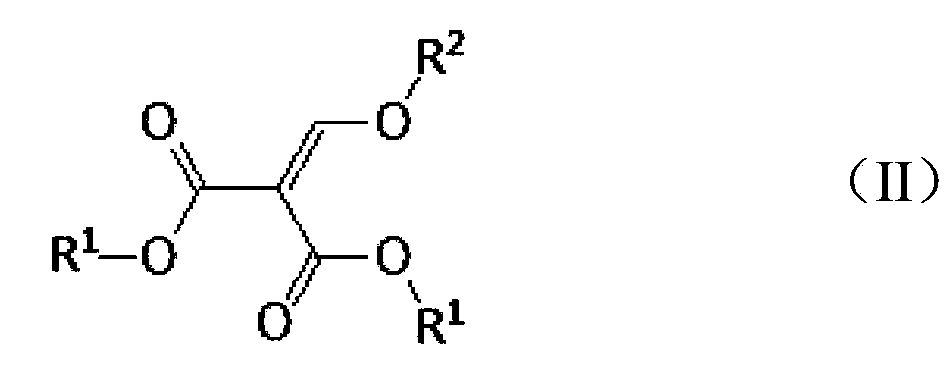
[0056] (1) Preparation of diethyl(3-bromoanilinomethylene)malonic acid
[0057] Add 30 g of analytically pure m-bromoaniline and 49 g of analytically pure diethyl ethoxymethylenemalonate into the reaction flask, stir, and heat slowly. When the reaction temperature rises to 110 ° C, stir for 1.5 h; after the reaction is completed, put The reaction solution is directly concentrated, and the ethanol generated by the reaction is removed, and the obtained thick product is purified by column chromatography, and after drying, 56.1 g of diethyl (3-bromoanilino methylene) malonic acid is obtained, and the calculated yield is 94%. The purity of diethyl (3-bromoanilinomethylene) malonic acid obtained by Agilent1100 liquid chromatography was 99.0%.
[0058] (2) Preparation of ethyl 7-bromo-4-hydroxy-3-quinolinecarboxylate
[0059] Under stirring, add 280 mL of diphenyl ether into the reaction flask, and when the temperature rises to 240°C, add 56.1 g of the above-prepared diethyl(3-bromo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com