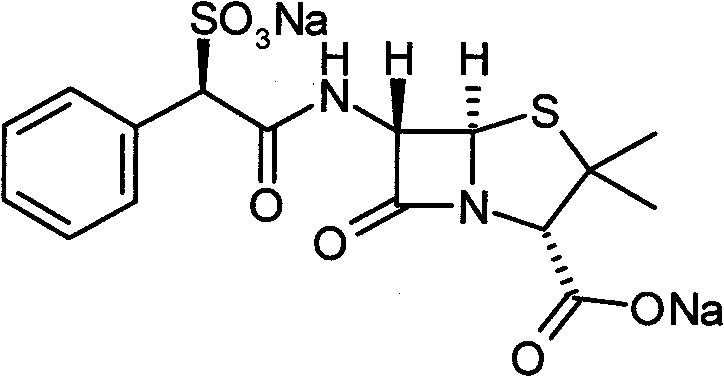
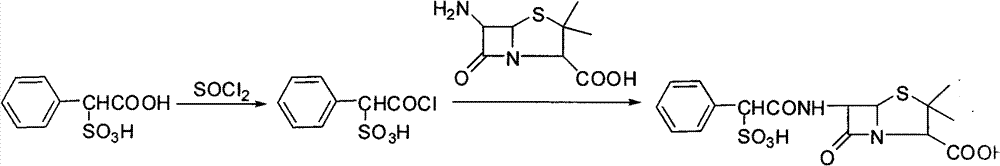
Preparation method of sulbenicillin sodium
A technology of sulfbenicillin sodium and sulfophenylacetic acid, which is applied in the direction of organic chemistry, can solve the problems of difficulty in improving product quality, instability of sulfophenylacetyl chloride, incomplete reaction, etc., and achieve excellent product quality, suitable for industrial production, The effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Dissolve 216g (1mol) sulfophenylacetic acid in anhydrous 1000ml dichloromethane, add 202.4g (2.2mol) propionyl chloride, cool to 0-5°C, add dropwise 449.5g (4.45mol) triethylamine, after completion React at 25°C for 3 hours, transfer the reaction solution into a separatory funnel, wash with water, extract with dichloromethane, dry and concentrate to obtain mixed anhydrides.
[0031] Weigh 202.3g (0.85mol) 6-APA and 243.3g (1.19mol) N, N-bis(trimethylsilyl)-2,2,2-trifluoroacetamide in the reaction flask, and nitrogen For protection, inject 1200ml of ethyl acetate as a solvent, raise the temperature to about 50°C and stir for 2 hours, after the reaction is completed, cool down to room temperature, wash with water, extract with ethyl acetate, and then dry and concentrate.
[0032] Use ethanol as a solvent, cool down to 0-5°C, slowly add step (1) to obtain mixed anhydride and 6-APA protected by step (2), keep warm for 45 minutes, acidify with hydrochloric acid, raise to 25°...
Embodiment 2
[0034] Dissolve 216g (1mol) sulfophenylacetic acid in anhydrous 1100ml dichloromethane, add 222.6g (2.1mol) butyryl chloride, cool to 0-5°C, add dropwise 454.5g (4.5mol) triethylamine, after completion React at 30°C for 3 hours, transfer the reaction solution into a separatory funnel, wash with water, extract with dichloromethane, dry and concentrate to obtain mixed anhydrides.
[0035] Weigh 202.3 grams (0.85mol) of 6-APA and 259.4 grams (1.27mol) of N, O-bis(trimethylsilyl)acetamide and put it into a reaction flask, vacuumize it and inject it into 1200ml of ethyl acetate to make The solvent was heated to about 50°C and stirred for 2 hours. After the reaction was completed, it was lowered to room temperature, washed with water, extracted with ethyl acetate, dried and concentrated.
[0036] Use ethanol as solvent, cool down to 0-5°C, slowly add step (1) to obtain mixed anhydride and 6-APA protected by step (2), keep warm for 30 minutes, acidify with hydrochloric acid, raise to...
Embodiment 3
[0038] Dissolve 216g (1mol) sulfophenylacetic acid in anhydrous 1200ml dichloromethane, add 300g (2.5mol) pentanoyl chloride, cool to 0-5°C, add dropwise 454.5g (4.5mol) triethylamine, after completion React at 30°C for 3 hours, transfer the reaction solution into a separatory funnel, wash with water, extract with dichloromethane, dry and concentrate to obtain mixed anhydrides.
[0039] Weigh 202.3 g (0.85 mol) of 6-APA and 219.5 g (1.36 mol) of hexamethyldisilazane into a reaction bottle, vacuumize and protect with nitrogen, inject 1350 ml of ethyl acetate as a solvent, and heat up to about 55 °C After stirring for 2 hours, the reaction was completed, cooled to room temperature, washed with water, extracted with ethyl acetate, dried and concentrated.
[0040] Use ethanol as a solvent, cool down to 0-5°C, slowly add step (1) to obtain mixed anhydride and 6-APA protected by step (2), keep warm for 50 minutes, acidify with hydrochloric acid, raise to 30°C, add sodium isooctanoat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com