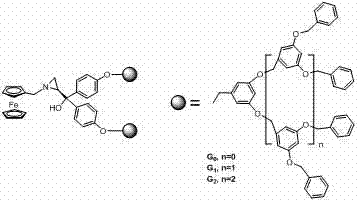
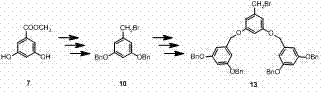
Ferrocenyl aza-annulet ligand loaded by tree-like macromolecule as well as synthetic method and application thereof
A technology of dendrimer and ferrocene methylene nitrogen, which is applied in the field of synthesis of ferrocene-based aza small ring ligands, can solve the problem of high cost of catalyst synthesis, and achieves low price, easy modification of price, and method. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
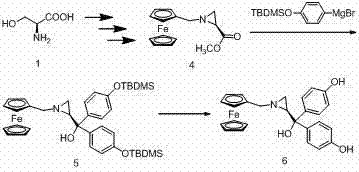
[0037] compound N - Synthesis of ferrocenemethyleneaziridine di-p-tert-butyldimethylsiloxyphenylmethanol 5:
[0038] Add anhydrous 20 mL THF, 0.53 g (22 mmol) magnesium chips, 5.7 g (20 mmol) p-tert-butyldimethylsilyloxybromobenzene into a 50 mL single-necked flask, add iodine particles and heat to reflux for 2 h. After the reaction is complete, cool to room temperature and add 0.5 g N - Methyl ferrocenemethylaziridine carboxylate. After the completion of the reaction traced by TLC, the reaction was terminated with saturated ammonium chloride. Then extract with ether, combine the organic phases, wash once with saturated NaCl aqueous solution, anhydrous NaCl 2 SO 4 dry. Diethyl ether was distilled off under reduced pressure, and the residue was purified by wet column to obtain 0.95 g (79%) of the product.
[0039] 1 H NMR (400 MHz, CDCl 3 ) δ 7.24 – 7.13 (m, 4H,), 6.78 – 6.70 (m, 4H,), 4.12 – 4.03 (m, 9H,), 3.71 (s, 1H,), 3.51 (d, J = 13.0 Hz, 1H,), 3.22 (d, J =...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com