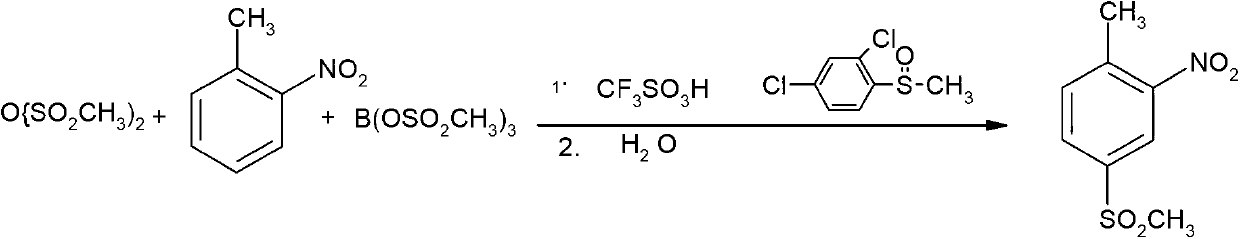
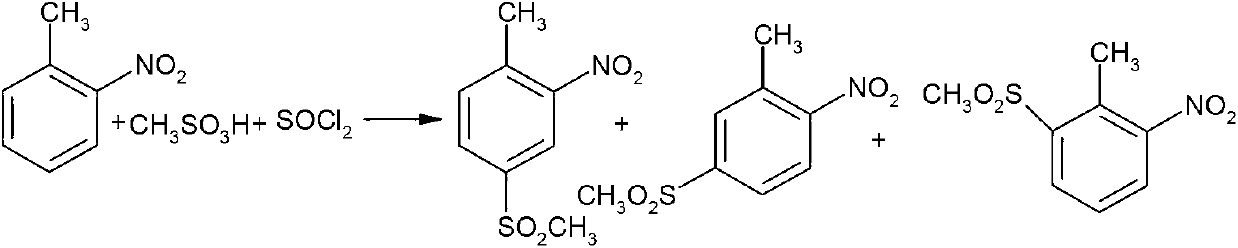
Synthesis method of weedicide mesotrione intermediate 2-nitro-4-mesyltoluene
A technology of mesyltoluene and mesotrione, which is applied in the fields of chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as unfavorable large-scale industrial production, achieve low cost, high yield, The effect of short synthetic routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 intermediate two 2-nitro-4-methanesulfonyl toluene
[0045] (1) Preparation of intermediate - 3-nitro-4-methylbenzenesulfonyl chloride
[0046]Add 130.8 grams of 98% chlorosulfonic acid (1.1 moles) into a 500 ml four-necked bottle equipped with a thermometer, agitator, reflux condenser and tail gas absorption device, heat up and keep it at 110-115 °C, and dissolve 137.8 grams of 99.5% chlorosulfonic acid Slowly drop o-nitrotoluene (1 mole) into the four-necked flask, and the dropwise addition is completed in about 3 hours. Then, the temperature of the reaction mixture is raised to 110-115°C, and the reaction is kept for 3 hours. The hydrogen chloride gas tail gas generated during the reaction is absorbed through the tail gas absorption system. Add 5 grams of N,N-dimethylformamide catalyst, keep 43-53 °C and add 138.2 grams of 99% thionyl chloride (1.15 moles) dropwise within 4-5 hours. After dropping, keep warm and control 60-65 °C After reacting for 3 ho...
Embodiment 2
[0049] Embodiment two, intermediate two 2-nitro-4-methanesulfonyltoluene
[0050] (1) Preparation of intermediate - 3-nitro-4-methylbenzenesulfonyl chloride
[0051] Add 142.5 grams of 98% chlorosulfonic acid (1.20 moles) into a 500 ml four-necked bottle equipped with a thermometer, stirrer, reflux condenser and tail gas absorption device, heat up and keep it at 110-115 ° C, and dissolve 137.8 grams of 99.5% Slowly drop o-nitrotoluene (1.0 mol) into the four-necked flask, and the dropwise addition is completed in about 3 hours. Then, the temperature of the reaction mixture is raised to 110-115°C, and the reaction is kept for 3 hours. The hydrogen chloride gas tail gas generated during the reaction is absorbed through the tail gas absorption system. Add 5 grams of catalyst N,N-dimethylformamide, keep 43-53 °C and add 133.8 grams of 99% thionyl chloride (1.125 moles) dropwise within 4-5 hours. After dropping, keep warm and control the reaction at 60-65 °C After 3 hours, HPLC m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com