Adsorbent, solid phase carrier and purification method
A purification method and solid-phase carrier technology, which can be used in peptide preparation methods, chemical instruments and methods, carrier binding/immobilization of peptides, etc., can solve problems such as ligand deterioration, and achieve prevention of modification, safe purification, high affinity The effect of harmony and resistance to deterioration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
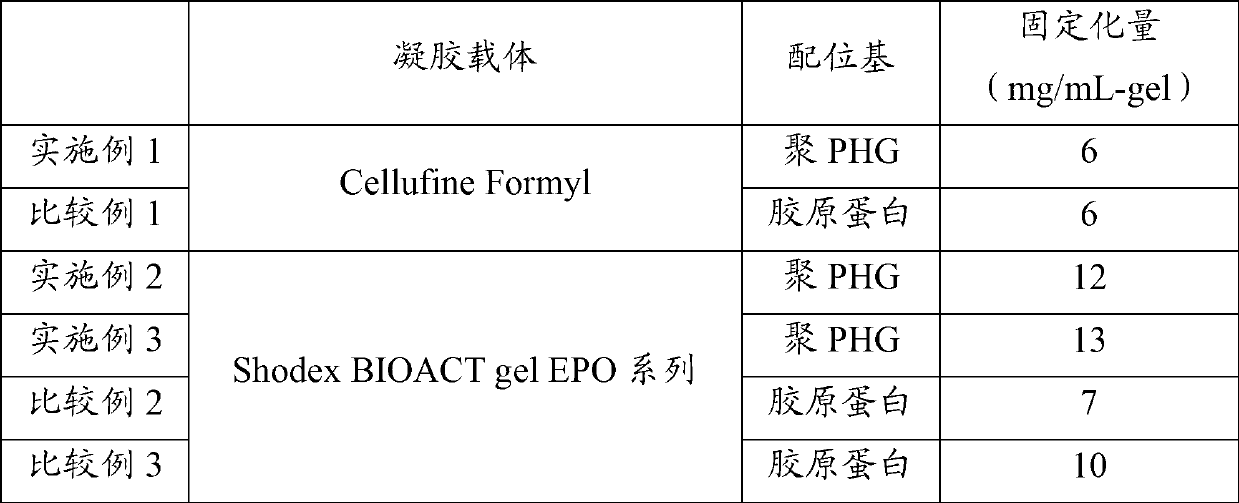
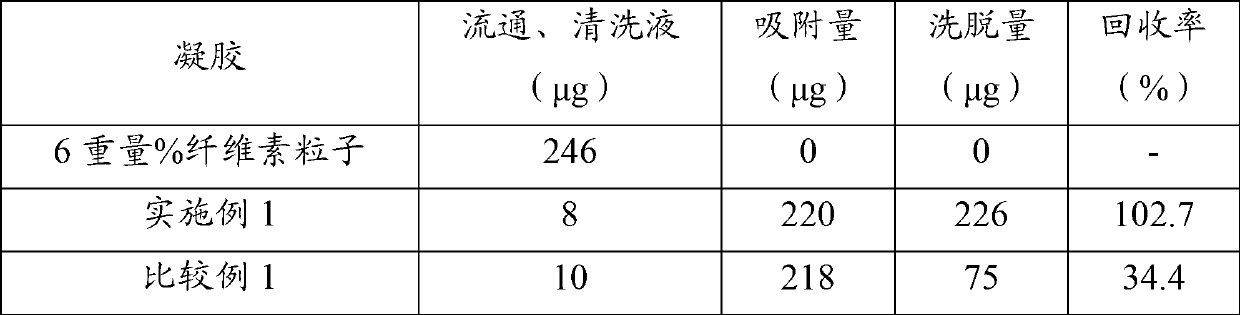
[0115] Cellufine Formyl (manufactured by JNC Co., Ltd.) was washed with 50 mM sodium carbonate and subjected to suction filtration, and 4.3 g of the obtained washed gel was placed in a 200 mL Erlenmeyer flask, and 16 g of an aqueous polypeptide solution containing 0.5% by weight of polyPHG was added. , 6.4 mL of 50 mM sodium carbonate, and 9.6 mL of water were thoroughly mixed, and shaken at 40° C. for 2 hours. 0.7 mL of an aqueous solution of 50 mg / mL of sodium borohydride was added, followed by further shaking at 40° C. for 2 hours. After the reaction, the reaction slurry was sucked and filtered to recover the gel. In order to remove unreacted substances remaining in the gel, the gel was washed with 50 mM sodium carbonate, aqueous hydrochloric acid (pH 3), and pure water, followed by suction filtration to obtain a polyPHG-immobilized gel.
[0116] The amount of polyPHG-containing polypeptide remaining in the washing liquid was quantified, and the amount of immobilized polyP...
Embodiment 2
[0122] Weigh 2.7 g of Shodex BIOACT gel EPO series (manufactured by Showa Denko Co., Ltd.) by dry weight, add to 30 mL of 0.25% by weight polyPHG-containing polypeptide, 50 mM sodium carbonate solution, and shake at 40° C. overnight reaction. After the reaction, the reaction slurry was sucked and filtered to recover the gel. In order to remove unreacted substances remaining in the gel, the gel was washed with 50 mM sodium carbonate, aqueous hydrochloric acid (pH 3), and pure water, followed by suction filtration to obtain a polyPHG-immobilized gel.
[0123] The amount of polyPHG-containing polypeptide remaining in the washing liquid was quantified, and the amount of immobilized polyPHG-containing polypeptide was calculated from the difference with the polyPHG-containing polypeptide used for the reaction. The amount of polyPHG-containing polypeptide in the washing solution was calculated by measuring the concentration with absorbance at 210 nm.
Embodiment 3
[0125] Except having changed reaction temperature into 4 degreeC from 40 degreeC, it carried out similarly to Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| composition ratio | aaaaa | aaaaa |
| weight-average molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com