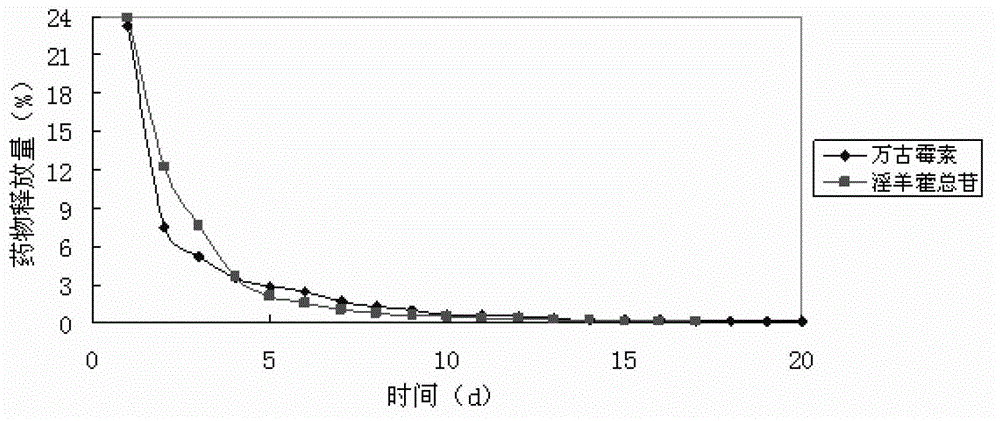
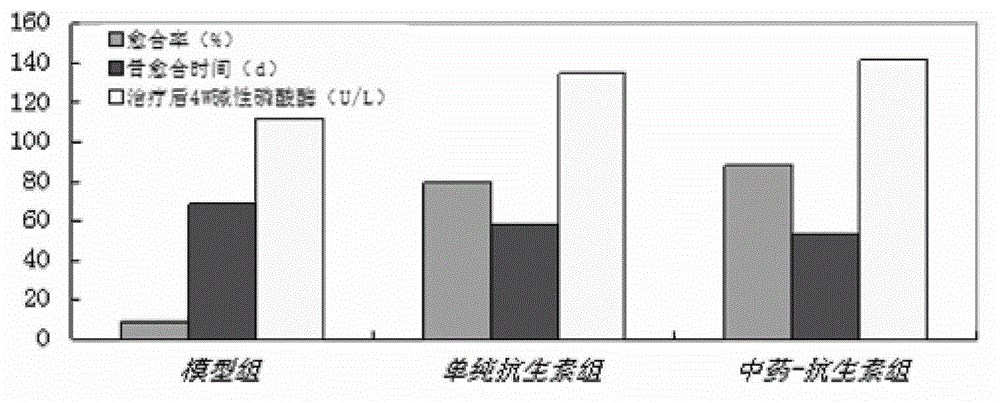
Implantable kidney-tonifying bone-invigorating traditional Chinese medicine extract-antibiotic-calcium sulfate sustained-release system, and preparation method and application thereof
A technology of invigorating the kidney and strengthening bones and antibiotics, which is applied in the directions of drug combinations, pharmaceutical formulations, plant raw materials, etc., to achieve the effects of improving the cure rate, reducing complications, and having good application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The operation steps of the preparation method of icariin-vancomycin-calcium sulfate sustained-release preparation are as follows:
[0046] 1) According to the proportion of the prescription, weigh 9.5g of calcium sulfate carrier, put it in a clean container, and set aside;
[0047] 2) According to the proportion of the prescription, weigh 0.12g of total icariin (the content of icariin is >10% after content determination) and set aside;
[0048] 3) According to the prescription ratio, weigh 0.88g of vancomycin hydrochloride powder injection, add it to the total icariin in step 2), mix well to form a mixture, and set aside;
[0049] 4) Draw 3ml of sterilized physiological saline, add it to the mixture in step 3), dissolve into a clear solution, and set aside;
[0050] 5) Take the solution in step 4), add it to the carrier material in step 1), stir quickly and evenly, and immediately transfer it to the corresponding mold with a spatula, fill it up and remove air bubbles, pl...
Embodiment 2
[0052] The operation steps of the preparation method of icariin-tobramycin-calcium sulfate sustained-release preparation are as follows:
[0053] 1) According to the proportion of the prescription, weigh 9.5g of calcium sulfate carrier, put it in a clean container, and set aside;
[0054] 2) According to the proportion of the prescription, weigh 0.12g of total icariin (the content of icariin is >10% after content determination), 0.6g of sodium alginate, mix well, and set aside;
[0055] 3) Take 3ml of tobramycin sulfate, add it to the mixture of icariin and sodium alginate in step 2), dissolve, and if necessary, conduct ultrasound to promote dissolution into a clear solution, and set aside;
[0056] 4) Take the solution in step 3), add it to the carrier material in step 1), stir quickly and evenly, and immediately transfer it to the corresponding mold with a spatula, fill it up and remove air bubbles, place it for 5-10 minutes, and take it out after it is completely cured; Ob...
Embodiment 3
[0058] The preparation of Example 1 is used for local implant administration of chronic osteomyelitis rabbit model, and the steps include:
[0059] 1) According to the proportion of the prescription, weigh 9.5g of calcium sulfate carrier, put it in a clean container, and set aside;
[0060] 2) According to the proportion of the prescription, weigh 0.12g of total icariin (the content of icariin is >35% after content determination) and set aside;
[0061] 3) According to the prescription ratio, weigh 0.88g of vancomycin hydrochloride powder injection, add it to the total icariin in step 2), mix well to form a mixture, and set aside;
[0062] 4) Draw 3ml of sterilized physiological saline, add it to the mixture in step 3), dissolve the clear solution, and set aside;
[0063] 5) Take the solution in step 4), add it to the carrier material in step 1), stir quickly and evenly, and immediately transfer it to the corresponding mold with a spatula, fill it up and remove air bubbles, p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com