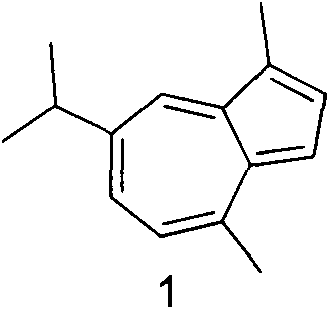
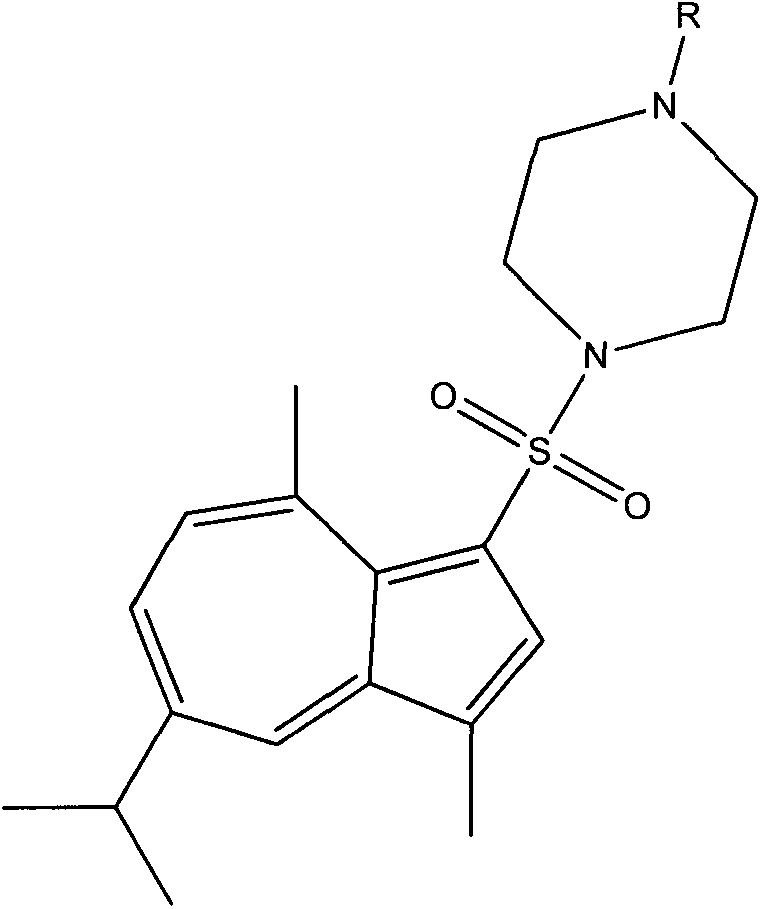
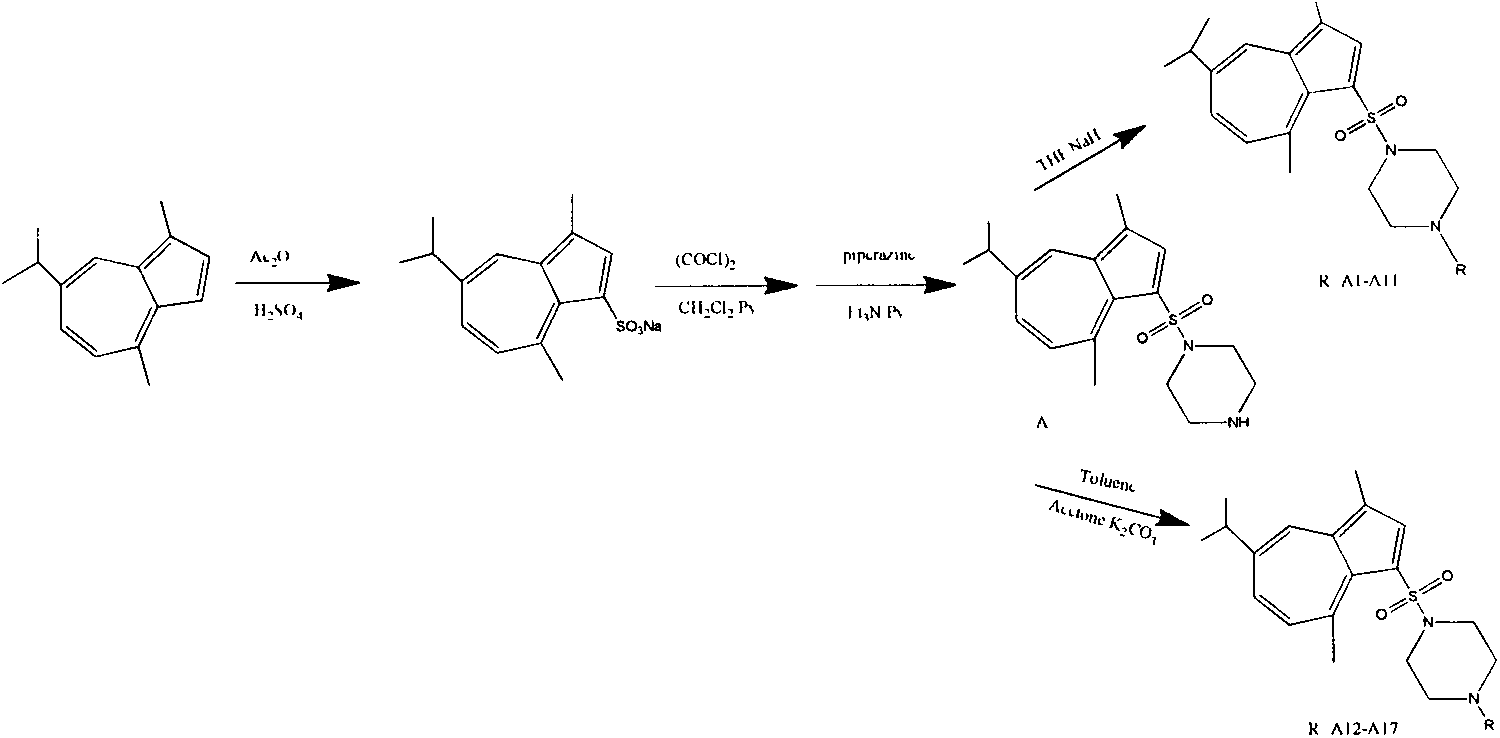
Synthesis of 1-substituent-5-isopropyl-3, 8-dimethyl azulenyl sulfonyl piperazine and anti-gastric ulcer activity research
A kind of technology of dimethylazulene sulfonylpiperazine and substituent, which is applied in the field of guaiazulene derivatives, and can solve problems such as increasing number of people
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1: This example prepares 5-isopropyl-3,8-dimethylazulenesulfonylpiperazine (abbreviated as A)
[0071]
[0072] The processing steps of the present embodiment are as follows:
[0073] Weigh azulene sulfonate sodium (1mmol) in a 25mL round bottom flask, add 10mL CH 2 Cl 2 , and add 3-5 drops of DMF and 0.5 mL of pyridine; add a small amount of CH to the dropping funnel 2 Cl 2 and add (COCl) 2 (2.5mmol), and then slowly added dropwise to the round bottom flask. After the reaction, slowly add 2 mL of Et 3 N, 1 mL of a mixture of pyridine and 3-bromoaniline (1.5 mmol). After the dropwise addition, react at room temperature for 1 hour, then add water corresponding to its volume to the reaction flask, adjust the pH to 5-6 with dilute hydrochloric acid, and 2 Cl 2 Extraction, the organic layer was washed with anhydrous Na 2 SO 4 Dry and spin dry, and the crude product is purified by column chromatography to obtain 5-isopropyl-3,8-dimethylazulenesulfonylpip...
Embodiment 2
[0074] Example 2: This example prepares 1-(benzenesulfonyl)-3,8-dimethyl-5-isopropyl-1-azulenesulfonylpiperazine (abbreviated as A1)
[0075]
[0076] The processing steps of the present embodiment are as follows:
[0077] Add 10ml of anhydrous THF to a 25mL pear-shaped bottle, then add 0.5g of NaH, and then add 0.2g of 1-substituent-5-isopropyl-3,8-dimethylazulenesulfonylpiperazine in which R is H , then add benzenesulfonyl chloride respectively, make it react at room temperature, the time varies, then add water equivalent to its volume in the reaction flask, extract with ethyl acetate and spin dry, and the crude product is purified by column chromatography to obtain 1-( Benzenesulfonyl)-3,8-dimethyl-5-isopropyl-1-azulenesulfonylpiperazine (purple crystal), yield 56.9%, m.p.158-160℃.IR(KBr): 3443, 2961 , 2930, 2842, 1543, 1445, 1355, 1327, 1305, 1264, 1174, 1149, 1095, 944, 898; 1 H NMR (400MHz, CDCl 3 )δ(ppm): 8.27(s, 1H), 8.01(s, 1H), 7.74(d, J=7.2Hz, 1H), 7.50~7.65(m...
Embodiment 3
[0078] Example 3: This example prepares 1-(p-toluenesulfonyl)-5-isopropyl-3,8-dimethyl-1-azulenesulfonylpiperazine (abbreviated as A2)
[0079]
[0080] The processing steps of the present embodiment are as follows:
[0081] Add 10ml of anhydrous THF to a 25mL pear-shaped bottle, then add 0.5g of NaH, and then add 0.2g of 1-substituent-5-isopropyl-3,8-dimethylazulenesulfonylpiperazine in which R is H , and then add p-toluenesulfonyl chloride respectively, make it react at room temperature, the time varies, then add water corresponding to its volume in the reaction flask, extract with ethyl acetate and spin dry, and the crude product is purified by column chromatography to obtain 1- (p-toluenesulfonyl)-5-isopropyl-3,8-dimethyl-1-azulenesulfonylpiperazine (purple crystal), yield 48.4%, m.p.123-125℃.IR(KBr): 3458 , 2962, 2360, 1635, 1455, 1332, 1311, 1270, 1147, 1096, 949, 727, 704; 1 H NMR (400MHz, CDCl 3 )δ(ppm): 8.27(s, 1H), 8.00(s, 1H), 7.57~7.63(m, 3H), 7.26~7.37(m, 3H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com