Dimethyl ether carbonylation catalyst, and preparation method and application thereof
A technology of carbonylation catalyst and dimethyl ether, which is applied in the field of catalytic chemistry and can solve the problems of catalyst carbon deposition and deactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the preparation of catalyst
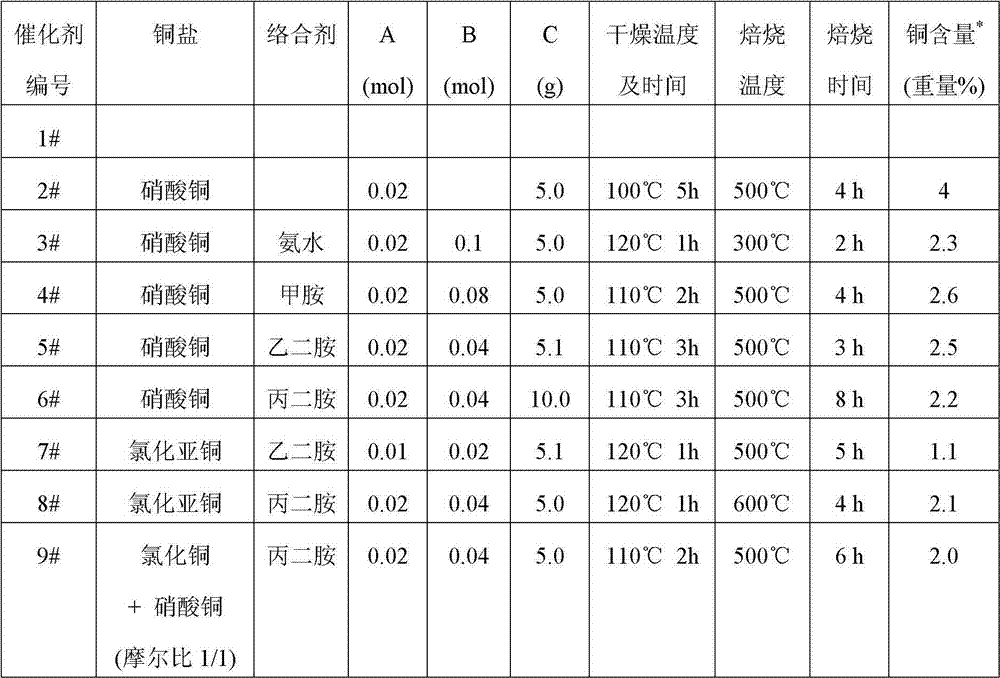
[0023] Weigh the copper salt of orientation Amol, add deionized 100ml, then add B mol complexing agent, stir evenly to form a copper complex solution, add Cg of hydrogen-type mordenite, stir evenly, the obtained solid sample is washed, After centrifugal separation, drying and roasting, the catalyst for the carbonylation of dimethyl ether to produce methyl acetate is obtained. The catalyst numbers and preparation conditions are shown in Table 1 below, wherein #1 is unmodified hydrogen mordenite.
[0024] Table 1
[0025]
[0026] *: Determined by XRF.
[0027] Note: the ammoniacal liquor adopts the ammoniacal liquor whose concentration of ammonia by weight is 25%, and its molar quantity in the table is calculated by the ammoniacal liquor in the ammoniacal liquor.
Embodiment 2
[0028] Embodiment 2: the reactivity performance of catalyst
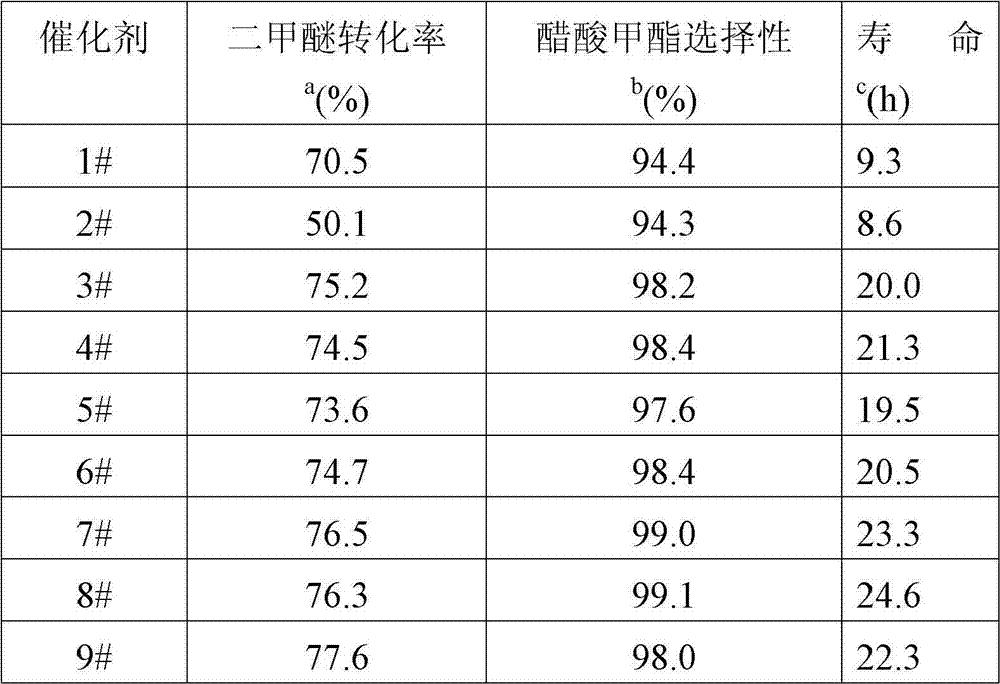
[0029] The 40-60-mesh sample obtained by tableting, crushing and sieving the catalyst powder obtained in Example 1 was used to measure the reaction performance of dimethyl ether carbonylation to produce methyl acetate. 0.6g of catalyst was loaded into the reactor, and a mixture of dimethyl ether, hydrogen, carbon monoxide and argon was passed through the reactor at a temperature of 220° C., a pressure of 30 atm, and a space velocity of 1500 ml / g / h. Among them, the gas flow rate F(H 2 +DME)=6.5ml / min, DME / H 2 =8.08 / 91.92 (volume ratio); gas flow F(CO+Ar)=8.5ml / min, CO / Ar=96 / 4 (volume ratio). The reaction results of each catalyst are listed in Table 2.
[0030] Table 2
[0031]
[0032] a: The highest conversion rate during the reaction.
[0033] b: Selectivity to methyl acetate when the highest conversion is reached during the reaction.
[0034] c: The time elapsed from the highest conversion rate to half of...
Embodiment 3
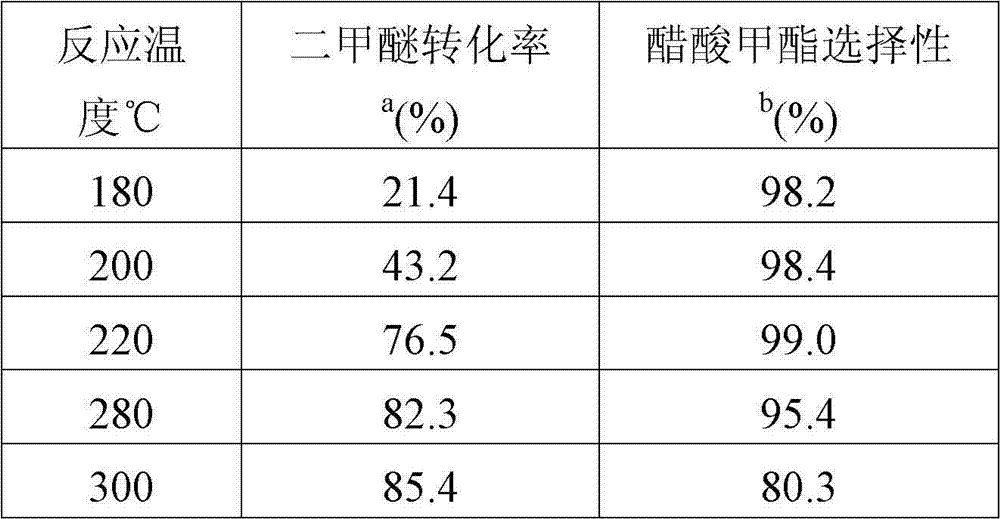
[0036] Reaction performance under different reaction temperatures on the 7# catalyst, other reaction conditions are the same with embodiment 2, only change reaction temperature. The reaction results are listed in Table 3.
[0037] table 3
[0038]
[0039] a: The highest conversion rate during the reaction.
[0040] b: Selectivity to methyl acetate when the highest conversion is reached during the reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com