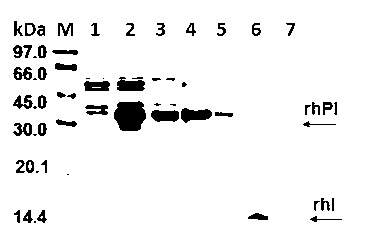Recombinant human proinsulin renaturation and purification method
A technology of recombinant human insulin and purification methods, applied in the preparation methods of insulin and peptides, chemical instruments and methods, etc., can solve problems such as the preparation of difficult protein drugs, and achieve simple and mature operation, easy control and amplification, and renaturation efficiency. The effect of improving the mass recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Preparation of denatured extract of recombinant human proinsulin
[0029] use E. coli As a host cell to express recombinant human proinsulin (rhPI) protein, the fermentation broth was centrifuged, the cells were disrupted, and buffer I (20 mmol / L PBS, 1 mmol / L EDTA, pH 7.4) and II (2.0 mol / L urea, 1 mmol / L EDTA, 1.0 mol / L NaCl, 20 mmol / L PBS, pH 7.4) After washing and centrifuging for crude purification, the obtained inclusion bodies were dissolved in 8.0 mol / L urea, 20 mmol / LDTT , 1.0 mmol / LEDTA, pH 8.0 (or 6-7 mol / L guanidine hydrochloride, 10-20 mmol / LDTT, 0.5-1 mmol / LEDTA, pH 7.0-8.0), the concentration of denatured extract is 9.92 mg / mL, the purity of rhPI is above 65.7%, see electrophoresis figure 1 Middle strip 3.
[0030] (2) Refolding and Purification of Recombinant Human Proinsulin by High Performance Size Exclusion Chromatography
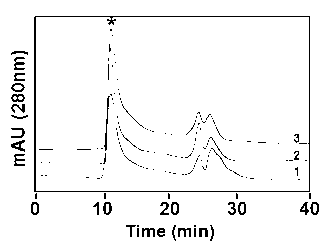
[0031] HPSEC column (TSK gel G2000SWXL, 300 × 7.8 mm I.D.) was equilibrated with mobile phase A (20 mmol / LPBS, 4.0 m...
Embodiment 2
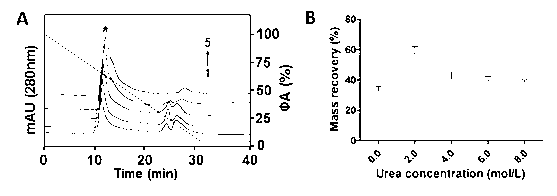
[0035] Similar to Example 1, the difference is that in step (2) equilibrate the TSK gel G2000SWXL HPSEC column (300 × 7.8 mm I.D.) with 100% mobile phase A without urea added, inject 200 μL of the denatured extract directly, and inject 200 μL of the denatured extract at a flow rate of 0.5 Perform linear gradient elution at mL / min for 30 min until the mobile phase B (20.0 mmol / L PBS, pH 6.5) is 100%, and extend for 10 min. The mass recovery rate of rhPI is 39.7%, and the purity can reach more than 95%. Chromatogram see figure 2 (A) Curve 1, mass recovery see figure 2 (B).
Embodiment 3
[0037]Similar to Example 1, the difference is that step (2) is equilibrated with 100% mobile phase A (20.0 mmol / LPBS, 2.0 mol / L urea, pH 7.0) under the condition of optimizing the urea concentration in the mobile phase and the chromatographic conditions TSK gel G2000SWXL type HPSEC column (300 × 7.8 mm I.D.), directly injected 200 μL of denatured extract, and carried out linear gradient elution of urea concentration for 30 min at a flow rate of 0.5 mL / min until mobile phase B (20.0 mmol / L PBS, pH 7.0) was 100%, extended for 10 min. The mass recovery rate of rhPI is 56.8%, and the purity can reach more than 96%. Chromatogram see figure 2 (A) Curve 2, mass recovery see figure 2 (B).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com