Preparation method of medical grade temperature sensitive chitosan blocking agent used for preventing leakage of cerebrospinal fluid
A temperature-sensitive chitosan technology, applied in the field of medical biomaterials, can solve problems such as large volume expansion and virus infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
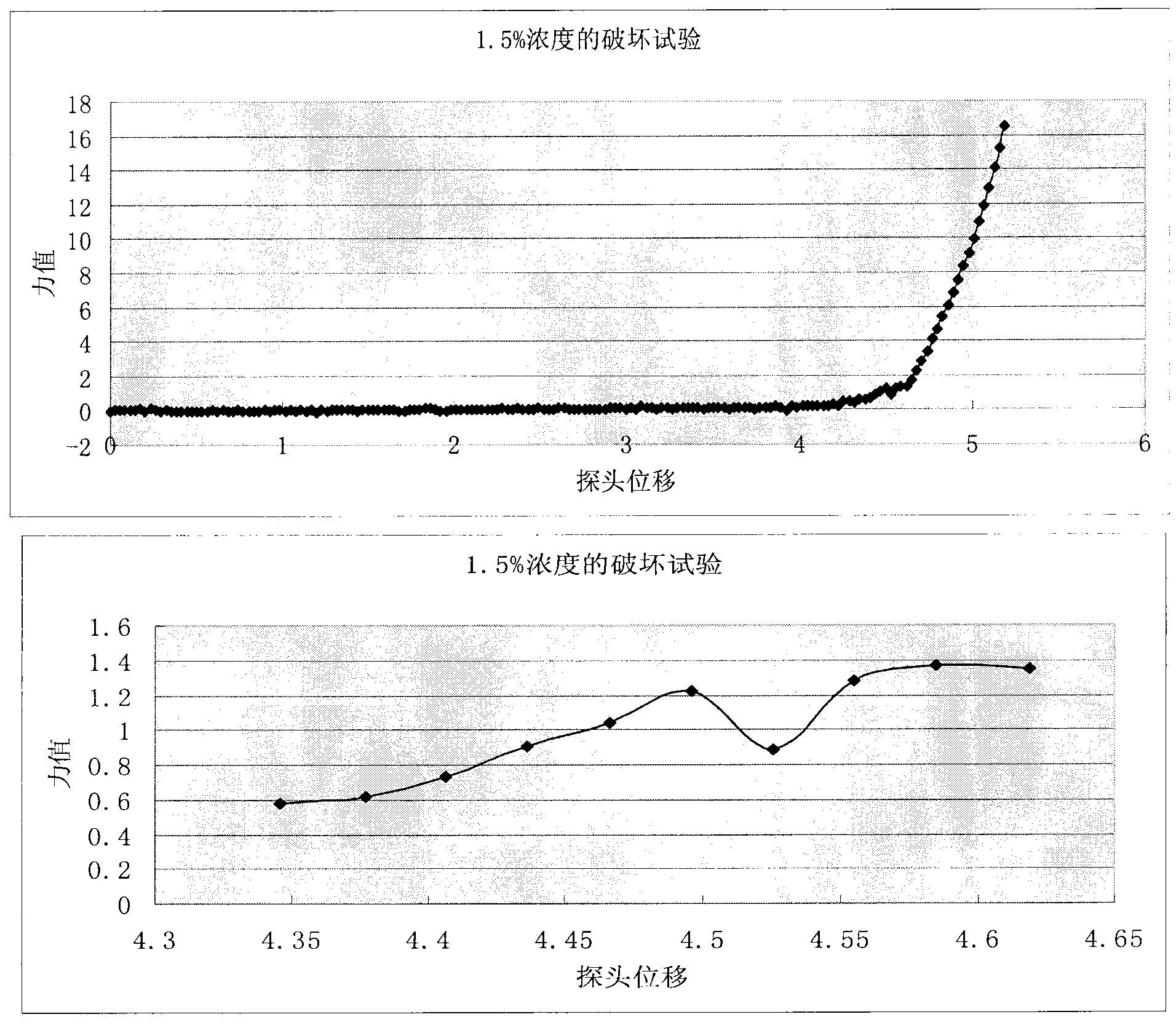
Image
Examples
Embodiment 1
[0039] Embodiment 1: the preparation of thermosensitive chitosan
[0040] In the first step, the chitosan with a degree of deacetylation of 50% is activated with potassium hydroxide with a mass fraction of 70% for 24 hours to become an activated form of hydroxy-K type.
[0041] The second step, hydroxybutylation of chitosan:
[0042] Filter and extrude excess lye in 10 grams of activated hydroxy-K chitosan with 100-mesh silk cloth, transfer to a three-necked bottle, add 100 ml of isopropanol and stir for 1-2 hours to completely disperse chitosan Open, then slowly add 100 ml of 1,2-epoxybutane dropwise with a separatory funnel, react at room temperature for 4-5 days, after the reaction is complete, use 8-10 times the volume of acetone to precipitate, wash, and vacuum dry at 50°C A white powder was obtained.
Embodiment 2
[0043] Embodiment 2: the preparation of thermosensitive chitosan
[0044] In the first step, the chitosan with a deacetylation degree of 30% is activated with potassium hydroxide with a mass fraction of 50% for 36 hours to the activated form of hydroxy-K type.
[0045] All the other steps are the same as in Example 1.
Embodiment 3
[0046] Embodiment 3: the preparation of thermosensitive chitosan
[0047] In the first step, the chitosan with a deacetylation degree of 30% is activated with potassium hydroxide with a mass fraction of 30% for 48 hours to become an activated form of hydroxy-K type.
[0048] All the other steps are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com