Reagent and method for improving reverse transcriptase performance
A reverse transcriptase and reagent technology, applied in the biological field, can solve problems that do not involve additive technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Wild-type M-MLV reverse transcriptase (Silo Bio, containing 1 mM DTT) was added to Reagent A described below at a volume ratio of 9:1. After mixing by pipetting for several minutes, the obtained enzyme solution was added to the B reagent described below at a volume ratio of 9:1.
[0057] Prepare A reagent according to the following formula:
[0058]
[0059]
[0060] Prepare B reagent according to the following formula:
[0061]
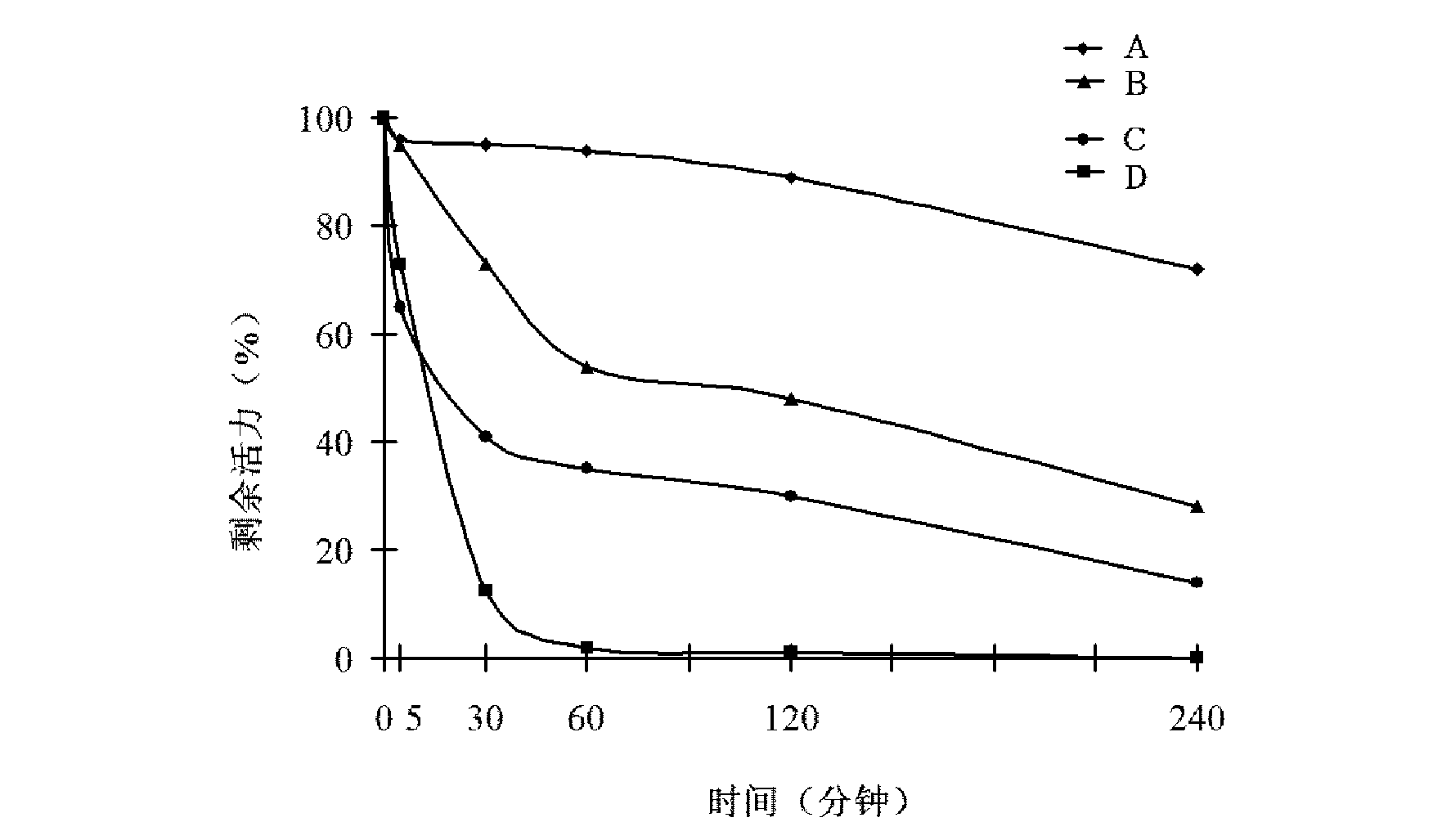
[0062] Taking the enzyme solution as a sample, the fluorescence real-time quantitative analysis system (Agilent, Mx3000p) and real-time quantitative PCR instrument (ABI, StepOne TM Real-Time PCR System), PCR instrument, electrophoresis instrument and other instruments, refer to the instruction manual, measure the reverse transcriptase RT activity and RNase H activity of the sample solution, and calculate the remaining activity. The results are shown in Table 1.
[0063] As can be seen from the results in Table 1, in the reagent of ...
Embodiment 2
[0067] Wild-type M-MLV reverse transcriptase (Silo Bio, containing 1 mM DTT) was added to Reagent A described below at a volume ratio of 9:1. After mixing by pipetting for several minutes, the obtained enzyme solution was added to the B reagent described below at a volume ratio of 9:1.
[0068] Prepare A reagent according to the following formula:
[0069]
[0070] Prepare B reagent according to the following formula:
[0071]
[0072] Taking the enzyme solution as a sample, the fluorescence real-time quantitative analysis system (Agilent, Mx3000p) and real-time quantitative PCR instrument (ABI, StepOne TM Real-Time PCR System), PCR instrument, electrophoresis instrument and other instruments, refer to the instruction manual, measure the RT activity and RNase H activity of the sample solution, and calculate the remaining activity. The results are shown in Table 2.
[0073] As can be seen from the results in Table 2, in the reagent of this example, after M-MLV was trea...
Embodiment 3
[0077] Wild-type M-MLV reverse transcriptase (Silo Bio, containing 1 mM DTT) was added to Reagent A described below at a volume ratio of 4:1.
[0078] Prepare A reagent according to the following formula:
[0079]
[0080] Taking the enzyme solution as a sample, the fluorescence real-time quantitative analysis system (Agilent, Mx3000p) and real-time quantitative PCR instrument (ABI, StepOne TM Real-Time PCR System), PCR instrument, electrophoresis instrument and other instruments, refer to the instruction manual, measure the RT activity and RNase H activity of the sample solution, and calculate the remaining activity. The results are shown in Table 3.
[0081] As can be seen from the results in Table 3, in the reagent of this example, after M-MLV was treated with iodoacetamide with a mother solution concentration of 25 mM (5 times the final concentration), RT activity was lost by 48%, and RNase H activity was completely lost. The test results show that the reagent in this...
PUM
| Property | Measurement | Unit |
|---|---|---|
| PCR efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com