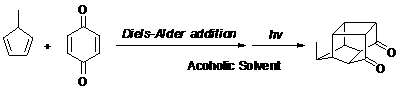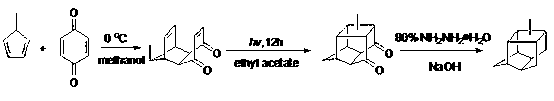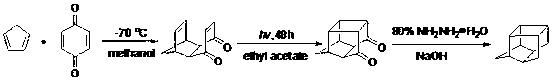Methyl pentacyclic undecanedione synthesis method
A technology for the synthesis of methylpentacycloundecanedione and its synthesis method, which is applied in the field of synthesis of methylpentacycloundecanedione, can solve the problems affecting the synthesis and application of MPCU compounds, complicated synthesis process and high cost, Achieve the effects of shortening the synthesis cycle, high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 108.0 g (1.0 mol) of p-benzoquinone to a 2000 ml three-necked flask, and add 1000 ml of methanol solvent. After the methanol solution of p-quinone was cooled to -40°C, 800.0 g (10.0 mol) of methylcyclopentadiene was added dropwise to the solution, the dropping rate was controlled at one drop per second, and stirring was started. After the dropwise addition, slowly return to room temperature. The reaction liquid after the addition was poured into a quartz glass test tube, and irradiated with ultraviolet light for 10 hours. After extraction and purification, 159.81 g of the product was obtained with a yield of 85%.
Embodiment 2
[0033] Add 54.0 g (0.5 mol) of p-benzoquinone to a 500 ml three-necked flask, and add 300 ml of ethanol solvent. After the ethanol solution of p-quinone was cooled to 20°C, 40.0 g (0.5 mol) of methylcyclopentadiene was added dropwise to the solution, the dropping rate was controlled at one drop per second, and stirring was started. After the dropwise addition was completed, it was slowly raised to room temperature. The reaction liquid after addition was poured into a quartz glass test tube, and irradiated with ultraviolet light for 40 hours. After extraction and purification, 85.5 g of the product was obtained with a yield of 91%.
Embodiment 3
[0035]Add 810.0 g (7.5 mol) of p-benzoquinone to a 2000 ml three-necked flask, and add 1000 ml of propylene glycol solvent. After cooling the propylene glycol solution of p-quinone to 0°C, add 60.0 g (0.75 mol) of methylcyclopentadiene dropwise to the solution at a rate of one drop per second and start stirring. After the dropwise addition was completed, it was slowly raised to room temperature. The reaction liquid after addition was poured into a quartz glass test tube, and irradiated with ultraviolet light for 30 hours. After extraction and purification, 129.7 g of the product was obtained with a yield of 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com