Imidazolyl ionic liquid, and synthesizing method and application thereof
A technology of ionic liquids and synthesis methods, applied in chemical instruments and methods, preparation of organic compounds, catalysts of organic compounds/hydrides/coordination complexes, etc., can solve the problems of poor recycling effects, catalyst poisoning, and high production costs of catalysts, etc. problems, to achieve the effect of wide range of applicable substrates, mild reaction conditions and excellent catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
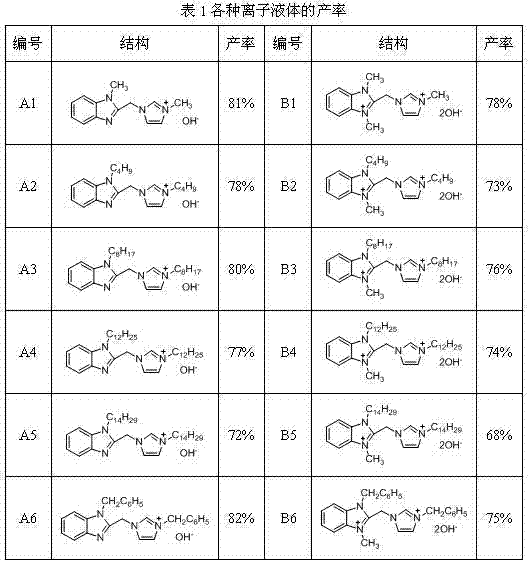
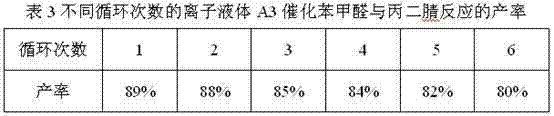
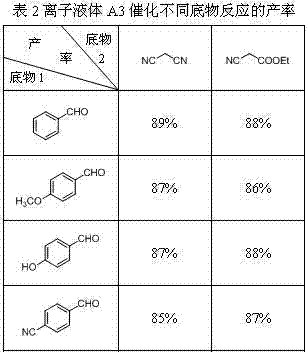
[0017] Embodiment 1: the synthesis of ionic liquid A3
[0018] Add 0.01mol 2-(1'-methylene imidazolyl)benzimidazole into 25mL of 25% sodium hydroxide solution, stir and react at 80°C, after the benzimidazole is completely dissolved, slowly add 0.01 mol n-C 8 h 17 Br, continue to react for 6 hours. After the reaction is complete, use a separatory funnel to separate the oil phase in the reaction solution, add it to 20mL of toluene, and slowly add 0.01mol n-C 8 h 17 Br, continue to react for 12 hours. After the reaction, the toluene was evaporated to obtain an oily liquid, which was purified by column separation. The purified oily liquid was added to 20 mL of dichloromethane, 0.01 mol of potassium hydroxide was added thereto, and the reaction was stirred at room temperature for 8 hours. After the reaction was completed, the precipitate was filtered off, and dichloromethane was distilled off to obtain the crude ionic liquid A3. The crude product was washed three times with ...
Embodiment 2
[0019] Embodiment 2: the synthesis of ionic liquid B3
[0020] Add 0.01mol 2-(1'-methylene imidazolyl)benzimidazole into 25mL of 25% sodium hydroxide solution, stir and react at 80°C, after the benzimidazole is completely dissolved, slowly add 0.01 mol n-C 8 h 17 Br, continue to react for 6 hours. After the reaction is complete, use a separatory funnel to separate the oil phase in the reaction solution, add it to 20mL of toluene, and slowly add 0.01mol n-C 8 h 17 Br, continue to react for 12 hours. After the reaction, the toluene was evaporated to obtain an oily liquid, which was purified by column separation. The purified oily liquid was added to 50 mL of toluene:methanol (v / v)=2:3 solution, and 0.05 mol of methyl iodide was slowly added dropwise thereto under reflux, and the reaction was continued for 12 hours. After the reaction was completed, the solid generated by the reaction was filtered out with suction, and the obtained solid was recrystallized from ethanol. Th...
Embodiment 3
[0021] Embodiment 3: the synthesis of ionic liquid A6
[0022] Similar to Example 1, the difference is: the raw material n-C 8 h 17 Br to C 6 h 5 CH 2 Cl, 3.25 g of pure ionic liquid A6 was obtained, and the yield was 82%. Mass spectrum (m / z): 379.20. NMR: 1 H NMR (400 MHz, CH 3 OD) δ: 9.06 (s, 1H), 7.74 (d, J = 6.8 Hz, 1H), 7.58 (dd, J = 5.2, 2.8 Hz, 3H), 7.48 – 7.36 (m, 7H), 7.33 – 7.29 (m, 3H), 7.05 (dd, J = 6.3, 2.6 Hz, 2H), 5.82 (s, 2H), 5.68 (s, 2H), 5.34 (s, 2H); 13 C NMR (101 MHz, CH 3OD) δ: 147.21, 141.39, 136.90, 135.86, 135.74, 133.40, 129.07, 129.05, 128.77, 128.54, 127.83, 125.96, 124.02, 123.19, 123.02, 122.53, 118.93, 110.58, 52.90, 46.60, 45.19。 Infrared (KBr): ν / cm -1 : 3449.42, 3061.24, 2968.84, 1623.20, 1562.36, 1452.64, 1332.61, 1161.06, 870.50, 745.70, 706.31, 641.15.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com