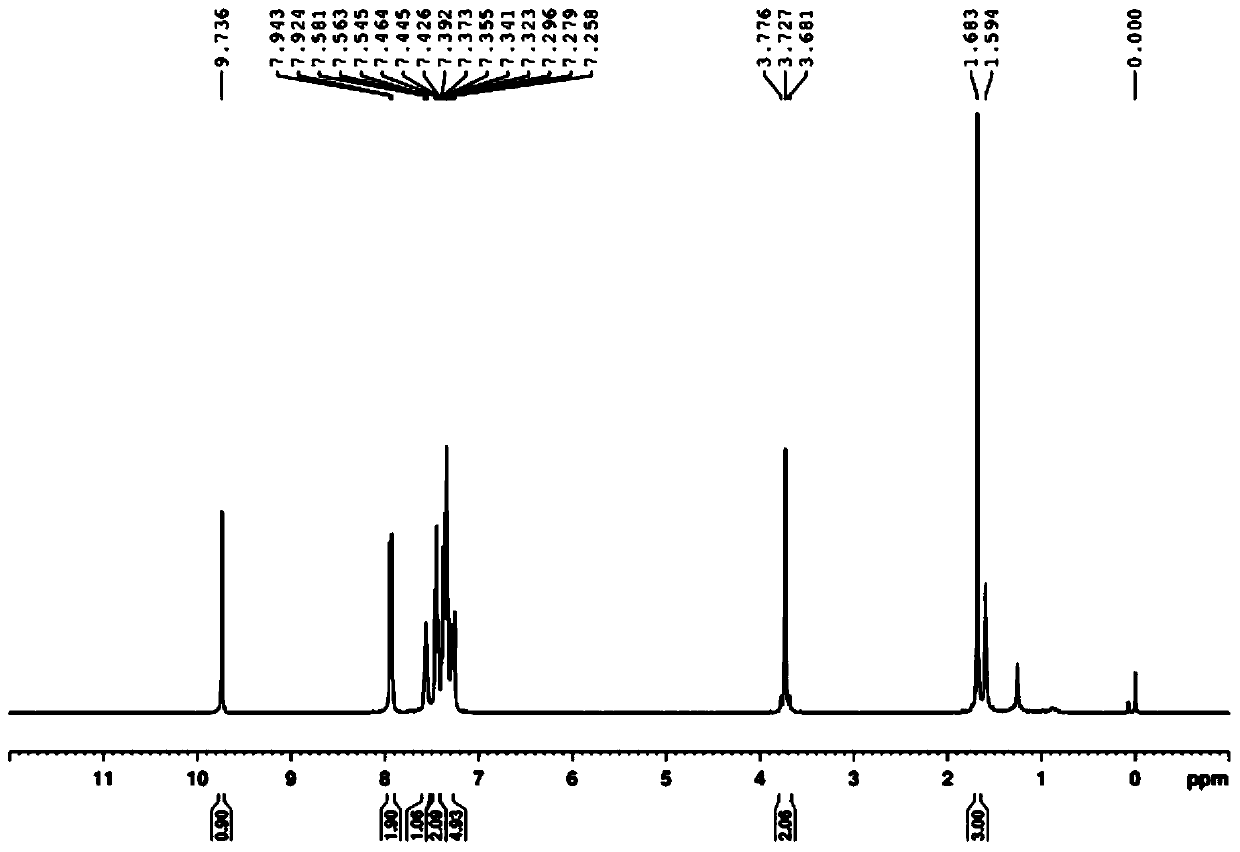
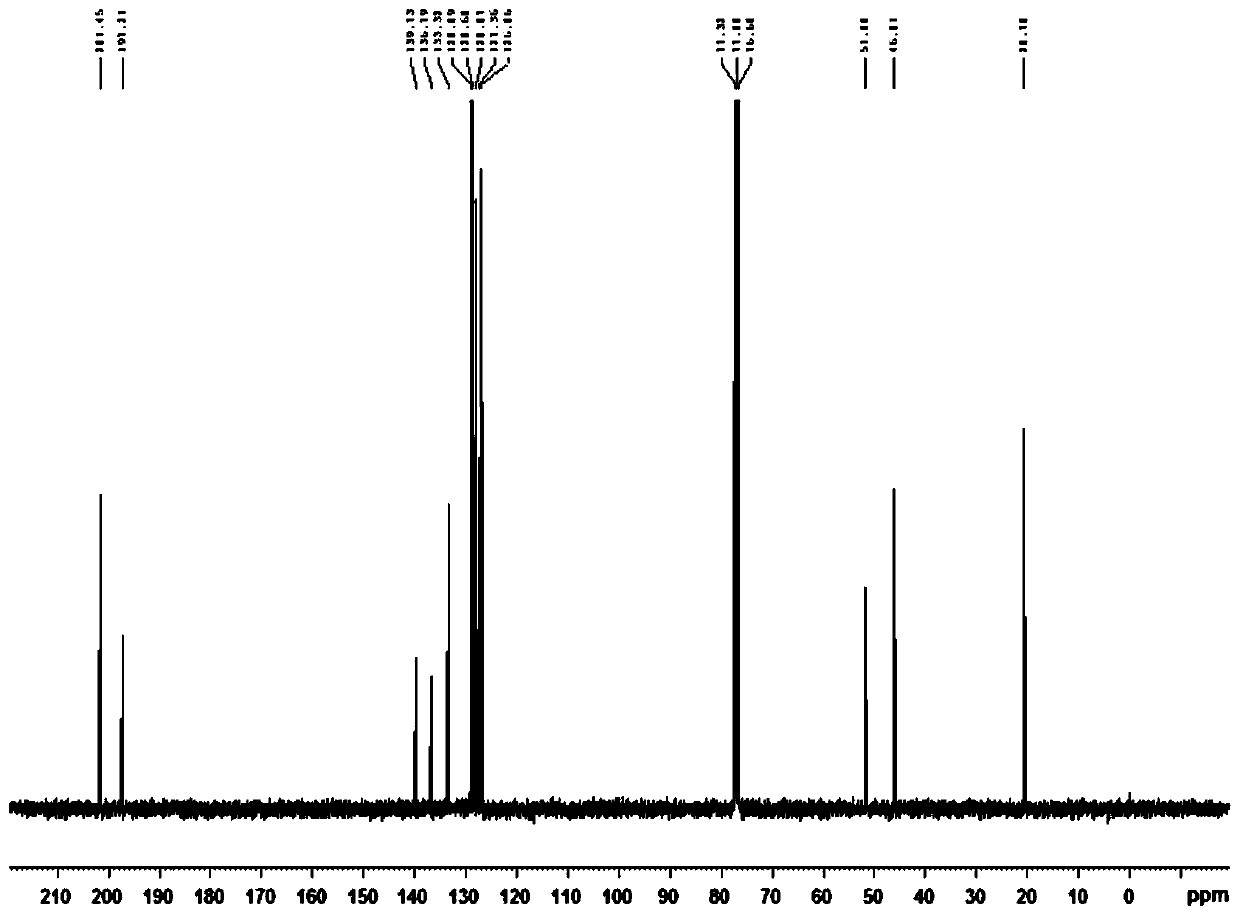
Synthesis of 2-methyl-4-carbonyl-2,4-diphenylbutyraldehyde
A technology of diphenylbutyraldehyde and synthesis method is applied in the synthesis of 2-methyl-4-carbonyl-2,4-diphenylbutyraldehyde and the synthesis field of 1,4-ketoaldehyde compound, which can solve the problem of expensive Catalysts, difficulties, harsh reaction conditions, etc., to achieve the effect of good tolerance, mild reaction conditions, and a wide range of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] To a solution of acetophenone (5.0 mmol, 1.0 equiv) in toluene (5.0 mL) was added 1,1-dimethoxy-N,N-dimethylmethylamine (7.0 mmol, 1.4 eq.), stirred And be warmed up to 110 ℃, after the completion of the reaction (monitored by TLC), quenched with water, extracted with ethyl acetate, dried with anhydrous sodium sulfate, then the dried reaction mixture was concentrated under reduced pressure, and passed through column chromatography (hexane Alkane: ethyl acetate = 1:1) to obtain (E)-3-(dimethylamino)-1-phenylprop-2-en-1-one.
[0026] A solution of p-toluenesulfonylhydrazide (5 mmol) in methanol (5 mL) was stirred at 60 °C until p-toluenesulfonylhydrazide was completely dissolved, then acetophenone was slowly added dropwise to the mixture, and a solid precipitate was obtained after standing , the solid precipitate was washed with petroleum ether to obtain p-toluenesulfonyl hydrazone, which was stored in vacuum for later use;
[0027] Using a one-pot method, mix (E)-3-(dim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com