Alkene-terminated polyfluorinated diaryl acetylene liquid crystal compound and preparation method thereof
A technology of polyfluorinated diaryl and polyfluorodiaryl groups, which is applied in the field of polyfluorodiarylacetylene-terminated liquid crystal compounds and their preparation, can solve the problem of high melting point and enthalpy of fusion, large dielectric anisotropy, Affecting the liquid crystal phase transition range and response speed, etc., to achieve the effect of high product yield and purity, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
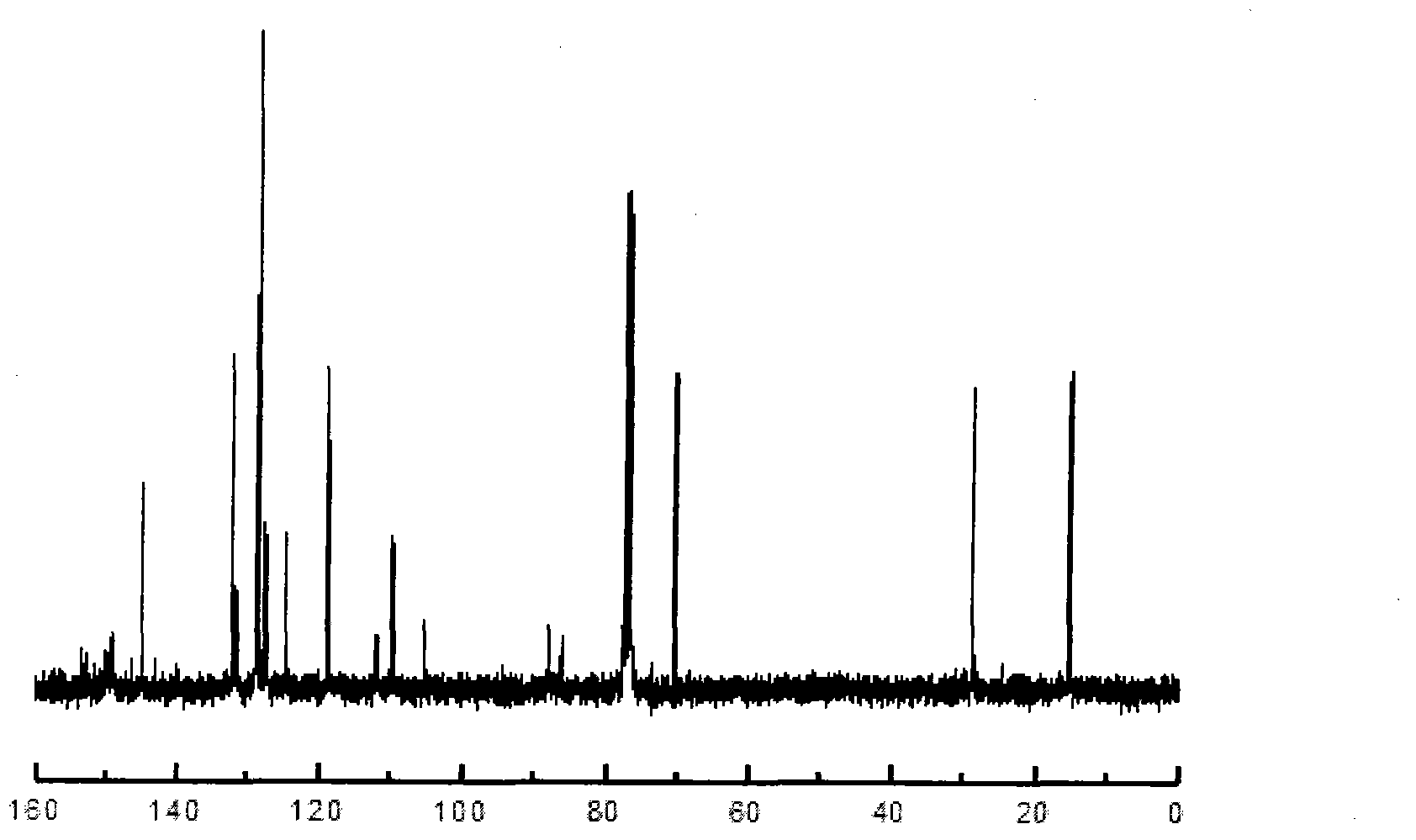
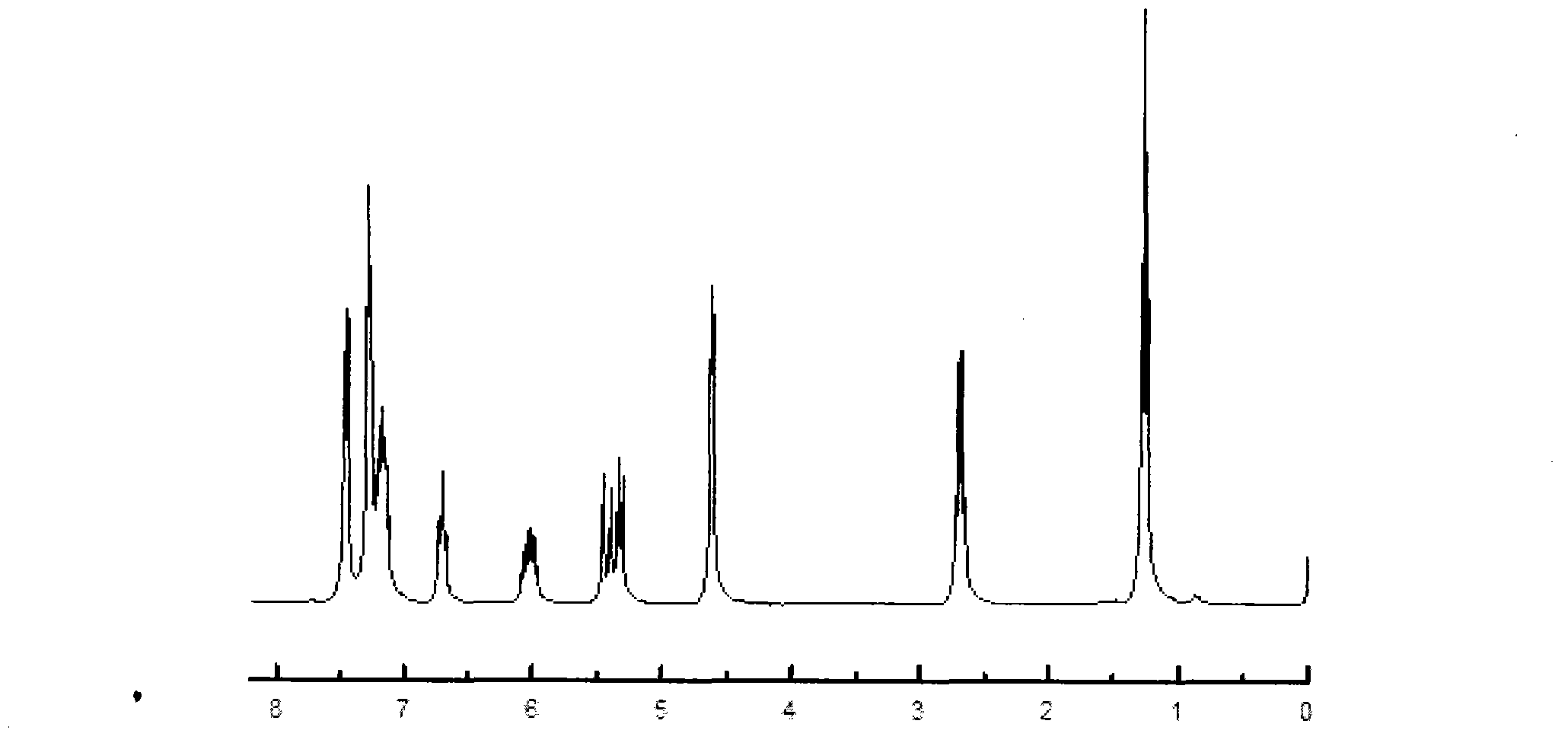
[0029] Take the preparation of 4-ethylphenyl-4'-allyloxy-2,3,2',3'-tetrafluorodiphenylacetylene as an example, wherein R is a straight-chain alkyl group of C2, and m and n are The values are all 0, and the value of x is 0. The raw materials used and their preparation methods are as follows:
[0030] Step 1: Synthesis of 4-ethylbenzeneboronic acid: under nitrogen protection, add 55.2g (0.30mol) of 4-ethylbromobenzene to a 500mL three-necked flask equipped with a constant pressure dropping funnel, a thermometer and a magnetic stirring bar, 250 mL of dry tetrahydrofuran was injected into the reaction flask and 144 mL (2.5 mol / L, 0.36 mol) of n-butyllithium solution was injected into the constant pressure funnel through a syringe, respectively. The reaction system was cooled to -78°C with liquid nitrogen, and the n-butyllithium solution was added dropwise. After the dropwise addition, the reaction was continued at this temperature for 1 h, and 69.0 g (0.30 mol) of tri-n-butyl bo...
Embodiment 2
[0044] Take the preparation of 4-propylphenyl-4'-allyloxy-2,3,2',3'-tetrafluorobenzil as an example, wherein R is a straight-chain C3 alkyl group, and the values of m and n are The values are all 0, and the value of x is 0, and the raw materials used and their preparation methods are as follows:
[0045] In Example 1, the 4-ethyl bromobenzene used was replaced with equimolar 4-propyl bromobenzene, and the other steps were the same as in the corresponding examples to prepare 4-propylphenyl-4'-allyloxy- 2,3,2',3'-Tetrafluorotoluene.
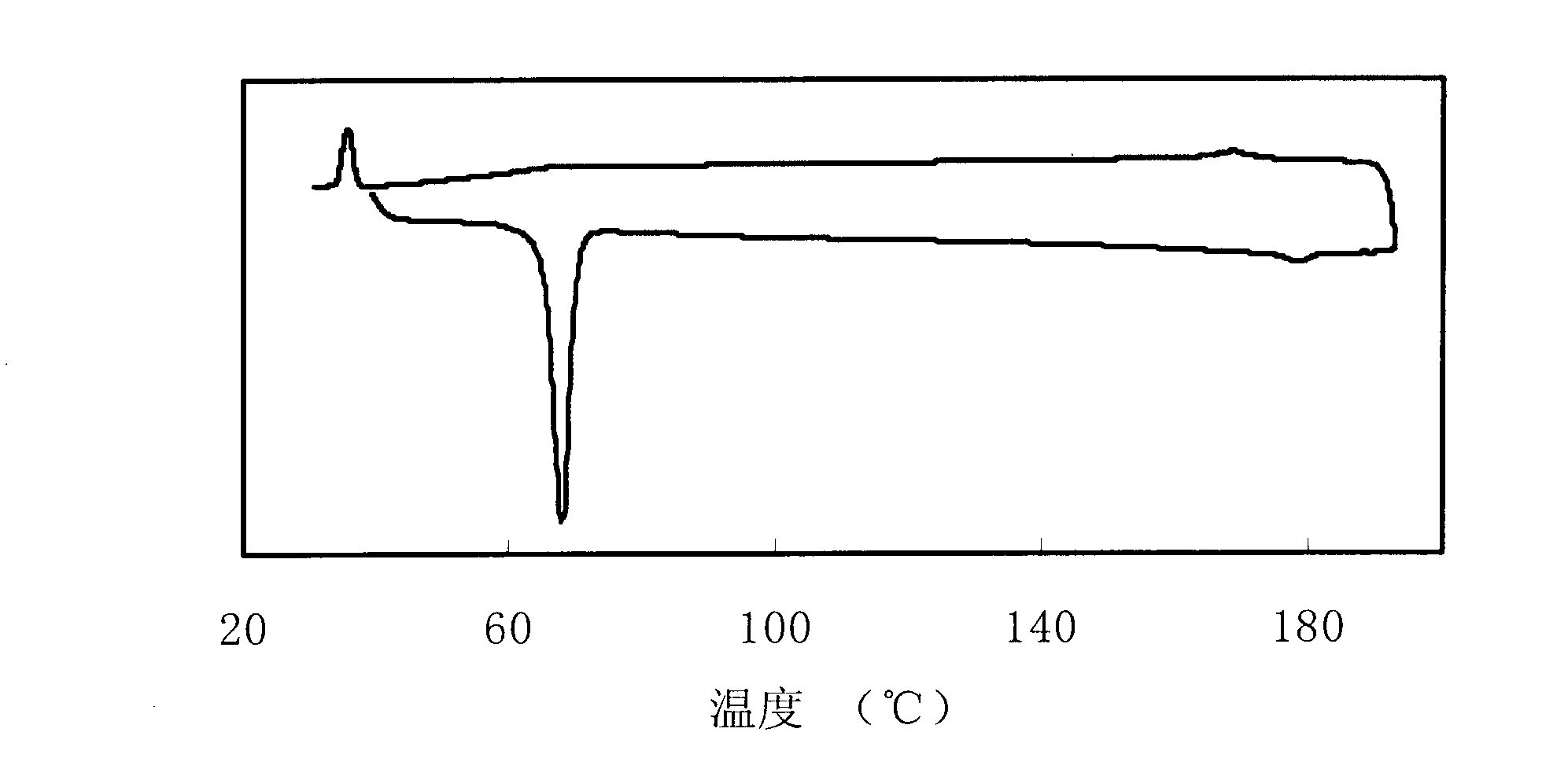
[0046] The phase transition temperature of 4-propylphenyl-4'-allyloxy-2,3,2',3'-tetrafluorobenzil is: Cr62.9N191.7I.
Embodiment 3
[0048] Take the preparation of 4-butylphenyl-4'-allyloxy-2,3,2',3'-tetrafluorotoluene as an example, wherein R is a C4 linear alkyl group, m and n are taken as The values are all 0, and the value of x is 0, and the raw materials used and their preparation methods are as follows:
[0049] In Example 1, the 4-ethylbromobenzene used was replaced with equimolar 4-butylbromobenzene, and the other steps were the same as in the corresponding examples to prepare 4-butylphenyl-4'-allyloxy- 2,3,2',3'-Tetrafluorobenzil.
[0050] The phase transition temperature of 4-butylphenyl-4'-allyloxy-2,3,2',3'-tetrafluorobenzil is: Cr40.6N168.2I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com