Preparation method and application for aminoglycoside-antibiotic molecularly imprinted polymers
A technology of aminoglycosides and molecular imprinting, which is applied in the field of analytical chemistry, can solve problems such as the preparation method of molecularly imprinted polymers that have not yet been seen in aminoglycoside antibiotics, and achieve the effect of high extraction recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1. Preparation method of aminoglycoside antibiotic molecularly imprinted polymer
[0021] (1) Dissolve 1-5mmol of streptomycin template molecules in 10mL of pure water, add 4-20mmol of functional monomer methacrylic acid, and stir the mixed solution at room temperature for 30min, so that the template molecules and functional monomers are fully Mix; then add 15-90ml of cross-linking agent ethylene glycol dimethacrylate and 20-100mL of methanol solvent; then add 5mmol of azobisisonitrile, and ultrasonically shake the resulting solution for 10min to make it evenly mixed, blow with nitrogen for 15min; The reaction solution was placed in a water bath at 60°C and stirred and heated at 1000 rpm for 24 hours to obtain a white solid polymer;
[0022] (2) After crushing and grinding the white solid polymer obtained in step (1) through a 75-mesh sieve, add it to a round-bottomed flask filled with 4M hydrochloric acid-methanol with a volume ratio of 1:1 and heat it under re...
Embodiment 2
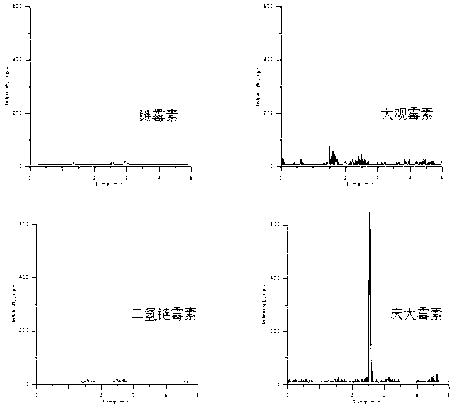
[0024] Example 2. MIP verification of the extraction effect of aminoglycoside antibiotics
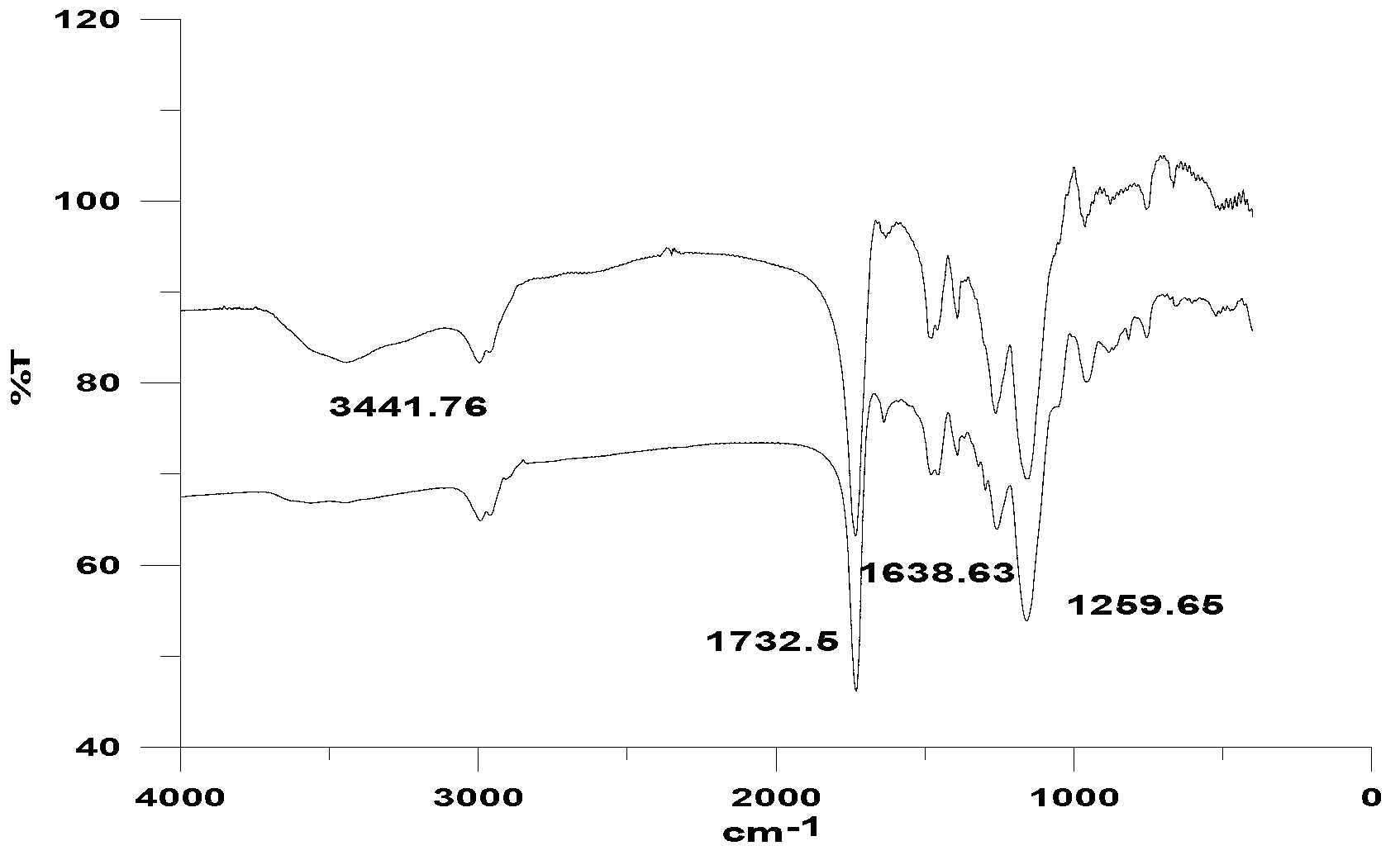
[0025] (1) After measuring the adsorption properties of molecularly imprinted polymers on template molecules at different ratios, the optimal ratio of template molecules: functional monomers: crosslinking agents = 1:14:60 was finally established. The resulting molecularly imprinted polymers (MIPs) were characterized by infrared spectroscopy, see figure 1 . It can be seen from the infrared spectrum that the materials before and after the eluted template molecules contain a wavenumber of 1638.63cm -1 It is a carbon-carbon double bond peak, and a wavenumber of 3441.76cm is added to the infrared spectrum of MIP after template removal -1 The peak, by 3441.76cm -1 、1732.5cm -1 、1259.65cm -1 These three peaks can confirm that carboxyl groups have been added in the eluted material structure, indicating that the template molecules in the obtained MIP material have been completely removed. ...
Embodiment 3
[0029] Example 3. MIP column detects commercially available honey
[0030] (1) Fill 200 mg of the molecularly imprinted polymer (MIP) obtained above into a 3 mL small column empty tube as a solid phase extraction (SPE) column filler to make an SPE small column. Activate with 3mL methanol and 3mL water first; then use a concentration of 20mmol / L K 2 HPO 4 The buffer solution (pH=7.4) was loaded, rinsed with 3mL water, and finally eluted with a mixture of FA / ACN=20 / 80 (v / v). The eluate was analyzed and detected by LC-MS / MS.
[0031] (2) Prepare standard solution: Prepare 4 μg / mL mixed standard solution with 0.1 mg / mL four aminoglycoside mycin stock solutions, and then prepare a series of standard concentration solutions step by step.
[0032] (3) Accurately weigh 2g of the blank honey sample, place it in a 15mL polypropylene plastic tube, and then add 5mL of it to a concentration of 20mmol K 2 HPO 4 The buffer solution was vortexed for 30 s, and then centrifuged at 4500 rpm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com