Preparation method and use of a nano-artificial antibody targeting cardiac troponin I
A cardiac troponin and artificial antibody technology, applied in the field of biomedicine, can solve the problems of large performance differences of monoclonal antibody batches, lack of selectivity and specificity, complex components of serum samples, etc., to overcome the long preparation cycle, high Effects of affinity, synthesis and simplicity of regeneration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of Cardiac Troponin I Artificial Antibody Based on Nanoparticle Three-Dimensional Structure Modification and Reconstruction Recognition Region
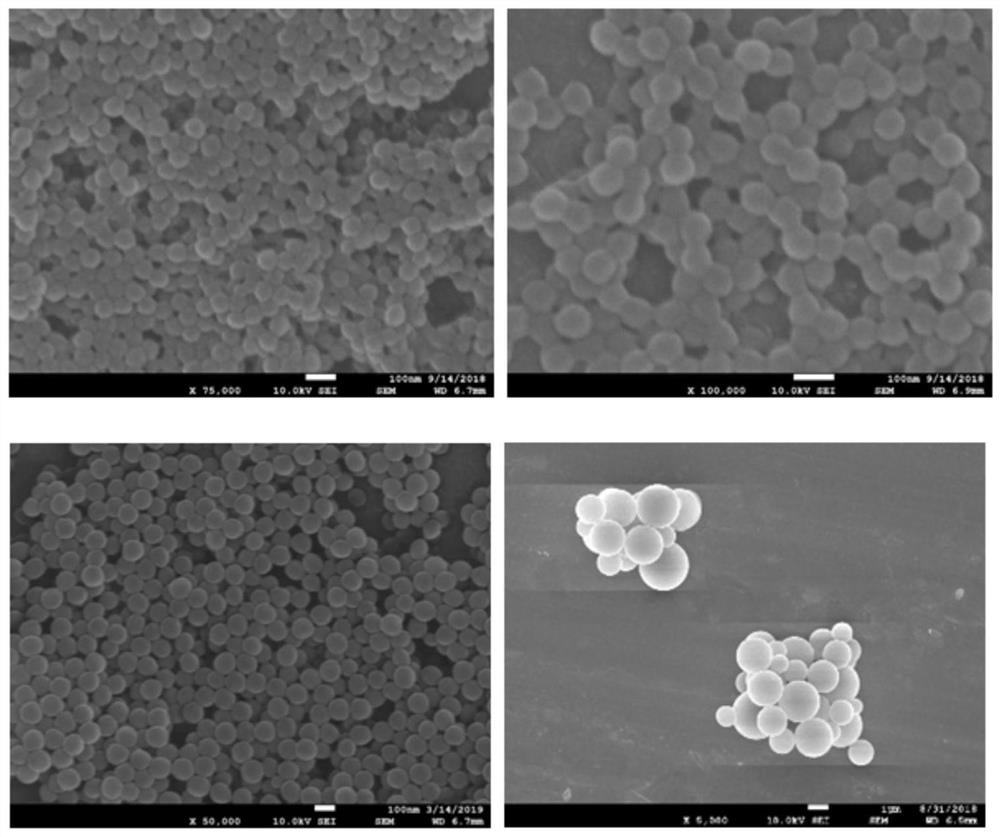
[0032] 1. Design and synthesis of nanoparticles
[0033] N-isopropylacrylamide (58-X mol%), charged monomer (3-acrylamidopropyl) trimethylammonium chloride (X mol%), N-tert-butylacrylamide ( 35 mol%), the crosslinker N,N'-methylenebisacrylamide (7 mol%) and sodium dodecylsulfonate (10 mg) were dissolved in water to give a total monomer concentration of 130 mM. After adding the initiator, polymerization was carried out at 65° C. for 3 hours with a magnetic stirrer under a nitrogen atmosphere. The polymerized solution was purified by dialysis against an excess of pure water, and polymer nanoparticles were obtained after freeze-drying.
[0034] 2. Preliminary screening of artificial antibodies
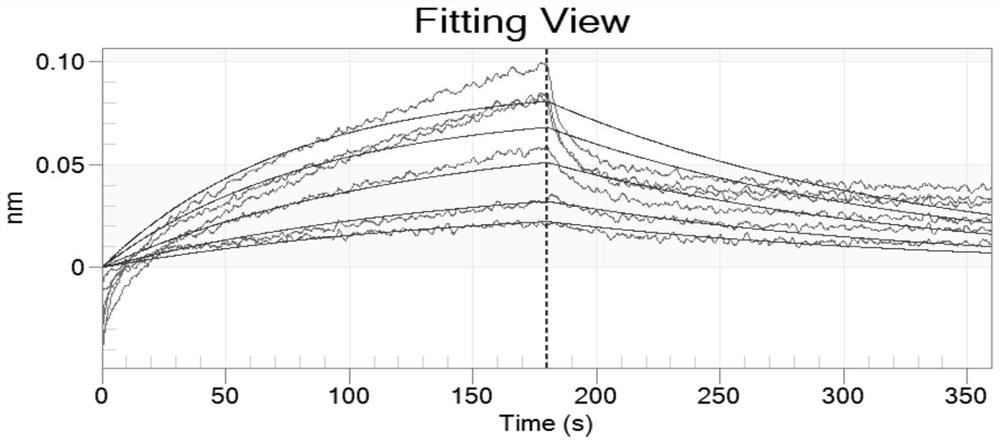
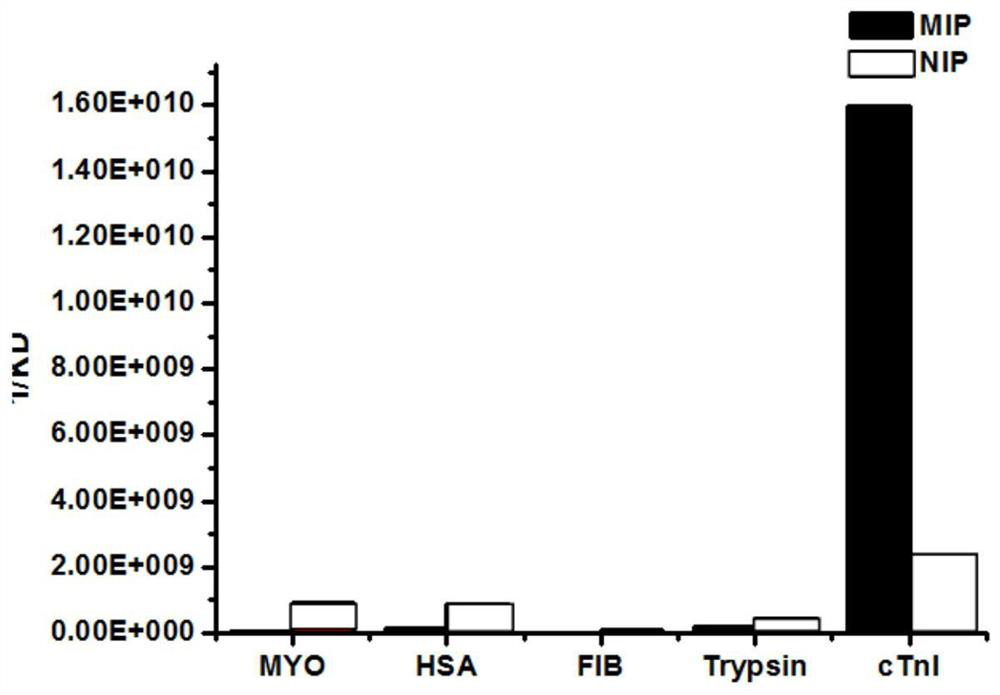
[0035] Troponin I was selected as the target, molecularly imprinted polymer nanoparticles were used as biomimet...
Embodiment 2
[0042] Example 2: Troponin I Nano-artificial Antibody as Ligand Combined with Monolithic Column Stationary Phase
[0043] First, a silane coupling agent needs to be bonded to the inner wall of the capillary column. Rinse the inner wall of the capillary column with acetone and pure water successively, pump through the capillary column with a sodium hydroxide solution with a concentration of 1 mol / L, so that it is fully and evenly filled with the capillary column, and then place it in an oven at 120°C . Then take out the capillary column that has completed the reaction, and rinse the sodium hydroxide in the capillary column with pure water until the pH of the solution pumped out of the capillary column is neutral. Then continue to wash with acetone and blow dry the capillary column with nitrogen, and put the capillary column in an oven at 120° C. without sealing to fully dry it. Dissolve 0.1 g of silane coupling agent 3-methacryloxypropyltrimethoxysilane in 0.9 g of anhydrous ...
Embodiment 3
[0045] Example 3: Nano-artificial antibody monolithic column realizes selective enrichment of low-abundance troponin in serum samples
[0046] Serum samples containing troponin I at a concentration of 0.001-10 μg / L were pumped into the monolithic column of nanoartificial antibodies through a microsyringe pump at a flow rate of 0.1-0.5 μL / min for selective enrichment, and after enrichment, 100 mM phosphoric acid was used to Buffer solution (pH=7) elutes impurity proteins non-specifically adsorbed on the monolithic column. Then the enriched troponin I was eluted, and the enrichment factor and recovery rate were calculated. Finally, the enrichment factor was 500, and the recovery rate was 98%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com