Divalproex sodium enteric-coated tablet core as well as preparation method and application thereof
A technology of divalproex sodium and enteric-coated tablets, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, active ingredients of anhydride/acid/halide, etc., which can solve the problem of unfavorable pressure granulation and unqualified product quality , high tablet content, etc., to achieve the effects of improving bioavailability in vivo, reducing hygroscopicity, and improving fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
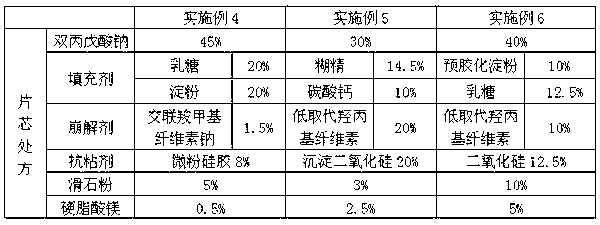
[0056] The composition of divalproex sodium enteric-coated tablet cores in Examples 1-3 is shown in Table 1.
[0057] Table 1 (composition of tablet core)
[0058]
[0059] The preparation method of divalproex sodium enteric-coated tablet core in embodiment 1-3, comprises the following steps:
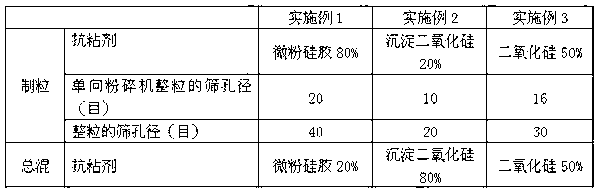
[0060] 1) Granulation: Divalproex sodium, fillers, disintegrants and 80% (w / w), 20% (w / w), 50% (w / w) of the total anti-adhesive agent After the adhesive is mixed evenly, it is put into a dry-compressed granulator for granulation; the obtained dry-compressed tablet is pulverized by a one-way pulverizer, passed through a 10-20 mesh sieve, and the obtained granules are granulated through a 20-40 mesh sieve;
[0061] 2) Blending and tableting: mix the sized granules with 20% (w / w), 80% (w / w), 50% (w / w) of anti-sticking agent, talcum powder, stearic acid After the magnesium is evenly mixed, it is pressed into tablets.
[0062] The process parameter of embodiment 1-3 is shown in table ...
Embodiment 4-6
[0066] The preparation process of embodiment 4 is the same as embodiment 1; the preparation process of embodiment 5 is the same as embodiment 2; the preparation process of embodiment 6 is the same as embodiment 3.
[0067] The process parameter of embodiment 4-6 is shown in table 3.
[0068] table 3
[0069]
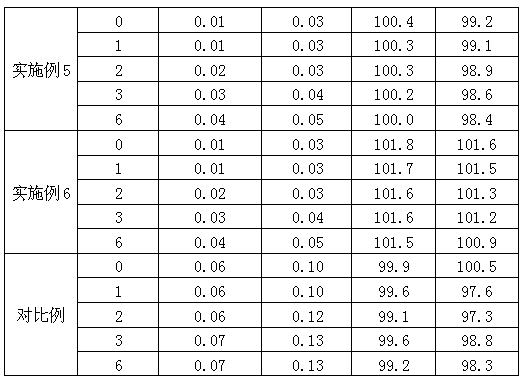
[0070] test case 1 Determination of Dissolution Profile of Divalproex Sodium Enteric-coated Tablets
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com