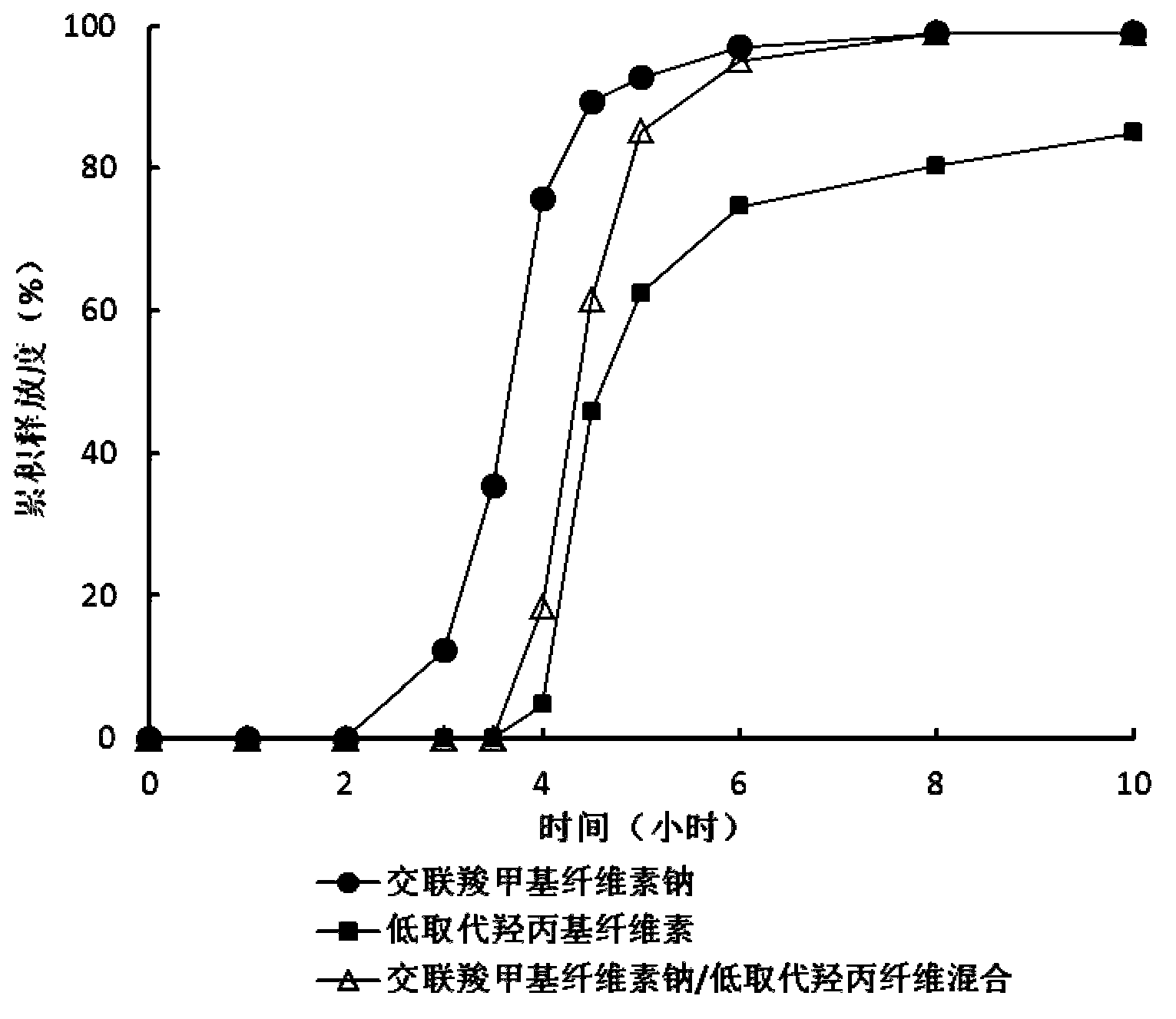
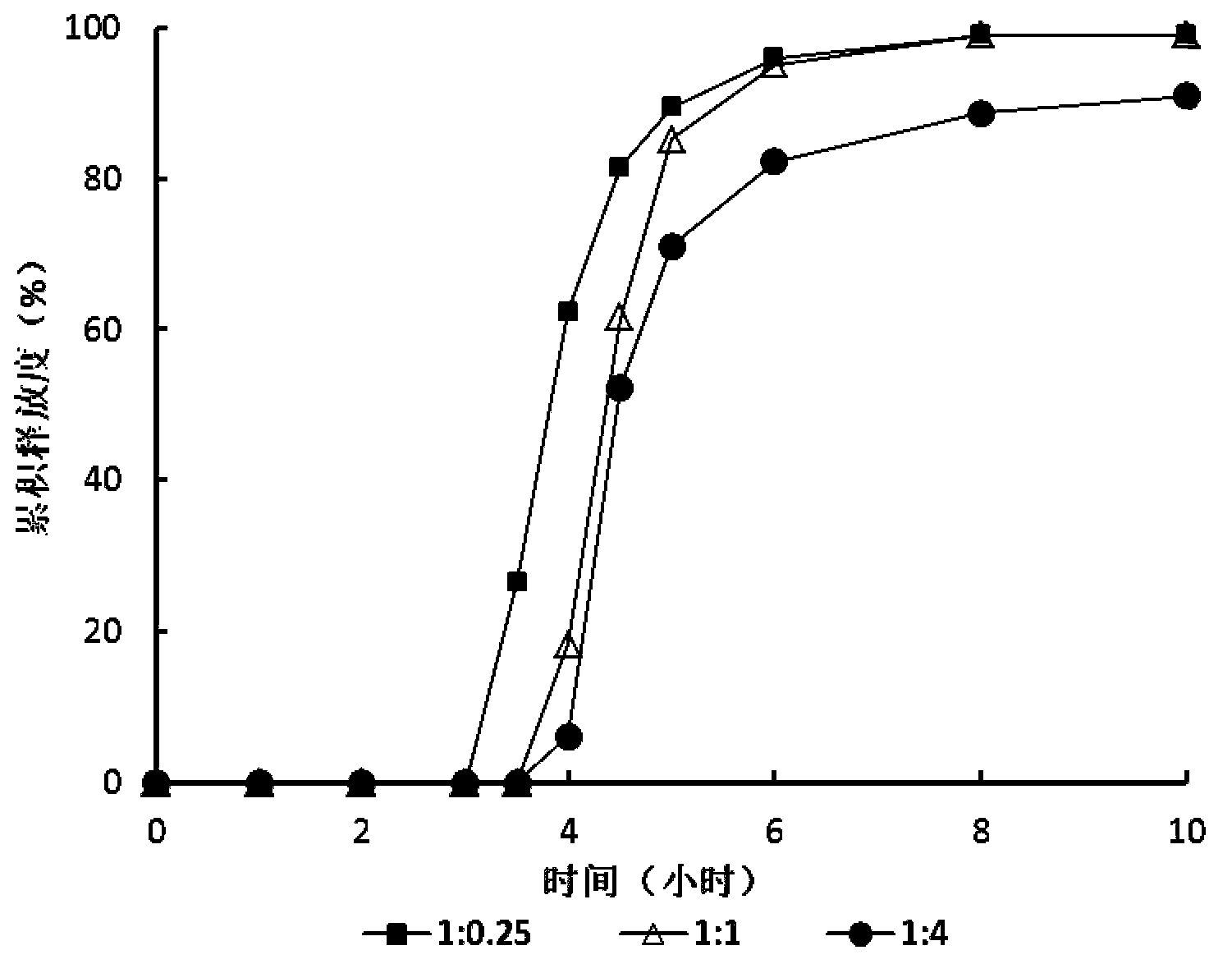
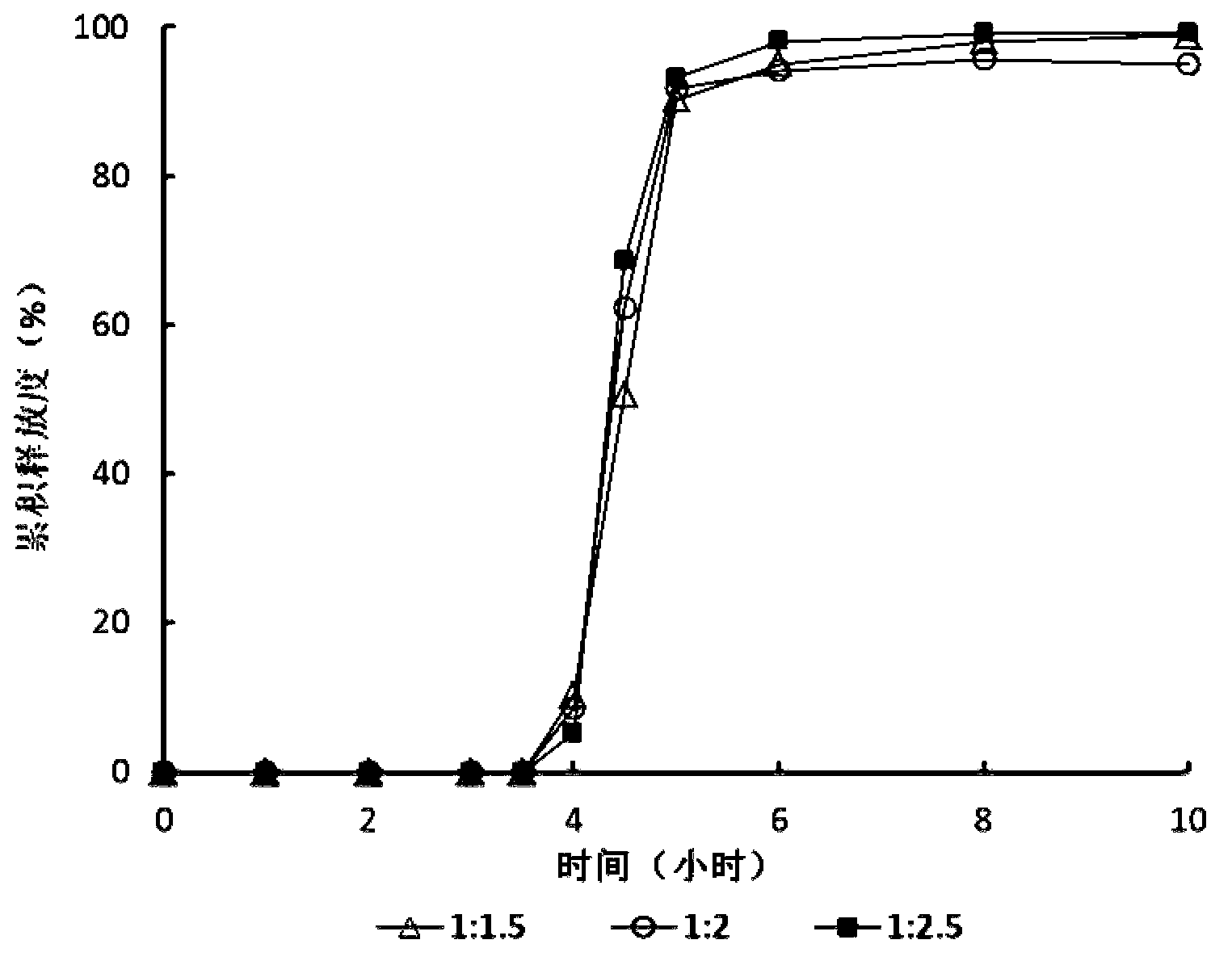
Zolpidem tartrate time-selecting pulse sustained-release pellet and preparation method thereof
A technology of zolpidem tartrate and pellets, which is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problem of unsatisfactory drug release rate, technological difficulty and slow drug release rate. and other problems, to achieve the effect of easy industrial production, simple preparation process, and reduction of the amount of auxiliary materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Drug-containing immediate-release pill core prescription:
[0041]
[0042] Preparation process: After mixing zolpidem tartrate and poloxamer 188, transfer it to a 60% ethanol solution of polyvinylpyrrolidone K30 and keep stirring. A fluidized bed is used to apply the drug by means of bottom spraying, and fluidized drying is continued at 40° C. for 2 hours after the drug application is completed, to obtain drug-containing pellets.
[0043] Swelling layer prescription:
[0044] CC-Na 8.5g
[0045] L-HPC 14.0g
[0046] Polyvinylpyrrolidone K90 3.0g
[0047] Preparation process: Disperse croscarmellose sodium and low-substituted hydroxypropyl cellulose in water, dissolve polyvinylpyrrolidone K90 in ethanol, and mix the two solutions to obtain a swelling layer coating solution. Fluidized bed coating is adopted, and a swelling layer is coated on the drug-loaded pellet core by a side spraying process.
[0048] Lag Layer Prescription:
[0049] Ethyl cellulose aqueous ...
Embodiment 2
[0052] Drug-containing immediate-release pill core prescription:
[0053]
[0054] Swelling layer prescription:
[0055] CC-Na 7.5g
[0056] L-HPC 16.0g
[0057] Polyvinylpyrrolidone K90 3.0g
[0058] Lag Layer Prescription:
[0059] Ethyl cellulose aqueous dispersion Surelease (based on solid content) 26.0g
[0060] The preparation method is the same as in Example 1.
Embodiment 3
[0062] Drug-containing immediate-release pill core prescription:
[0063]
[0064] Swelling layer prescription:
[0065] CC-Na 7.0g
[0066] L-HPC 17.0g
[0067] Polyvinylpyrrolidone K90 4.0g
[0068] Lag Layer Prescription:
[0069] Ethyl cellulose aqueous dispersion Surelease (based on solid content) 25g
[0070] Preparation process: with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com