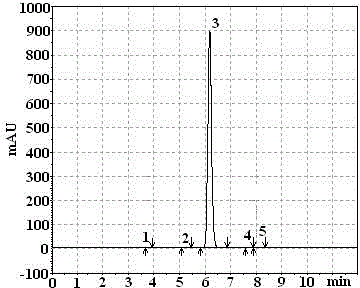
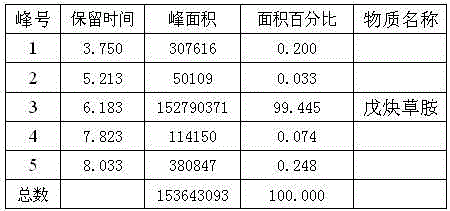
Synthetic method of propyzamide
A synthetic method, the technology of pentynclochlor, which is applied in the field of synthesis of pentynclod, can solve the problems of harsh production environment, high cost of raw materials, long reaction time, etc., and achieve improved process safety, low production cost and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Add 300g of chloroform and 100g of ethanol to 100.0g of benzonitrile (content 99%), and then add 1209.4g of sodium hypochlorite (content 13%), use 37% hydrochloric acid to keep the pH of the system at 3.0-4.0, at 55-60 At ℃, react until the content of 3,5-dichlorobenzonitrile is 99.5%, then add 30% sodium hydroxide under normal pressure to keep the pH value of the reaction system at 12-13, and keep the reaction at 80-90℃ After 2.5 hours, acidify the system with 37% hydrochloric acid until the pH value of the system is 1.0, react at 50-60°C for 1.0h, filter again, and dry at 90-110°C to obtain 182.5g of 3, with a content of 99.0%. 5-Dichlorobenzoic acid.
[0037] Take 100g of the obtained 3,5-dichlorobenzoic acid and mix it with 74.7g of thionyl chloride (content 99%), reflux at 70-80°C for 2.0h, and then recover the chlorine at normal pressure and 75-82°C sulfoxide, and then distill 3,5-dichlorobenzoyl chloride at 185-195°C and -0.01MPa for the next reaction.
[0038]...
Embodiment 2
[0040] Add 1500g chloroform and 500g ethanol to 100.0g benzonitrile (content 99%) respectively, then add 1209.4g sodium hypochlorite (content 13%), use 98% sulfuric acid to keep the pH value of the system at 5.0-6.0, at 60-70 At ℃, react until the content of 3,5-dichlorobenzonitrile is 99.4%, and then add 20% sodium hydroxide under normal pressure to keep the pH value of the reaction system at 11-12, and keep the reaction at 80-90℃ After 3.0 hours, acidify the system with 37% hydrochloric acid until the pH value of the system is 0, react at 50-60°C for 0.5h, filter again, and dry at 90-110°C to obtain 182.5g of 3, with a content of 99.0%. 5-Dichlorobenzoic acid.
[0041] Take 100g of the obtained 3,5-dichlorobenzoic acid and mix it with 74.7g of thionyl chloride (content 99%), reflux at 70-80°C for 2.5h, and then recover at normal pressure and 75-82°C Thionyl chloride, then distill 3,5-dichlorobenzoyl chloride at 185-195°C and -0.05MPa for the next reaction.
[0042] Take 52...
Embodiment 3
[0044] Add 75g of chloroform and 25g of ethanol to 100.0g of benzonitrile (content 99%), and then add 1209.4g of sodium hypochlorite (content 13%), use 25% hydrochloric acid to keep the pH value of the system at 0-1.0, at 55-70 At ℃, react until the content of 3,5-dichlorobenzonitrile is 99.5%, then add 10% sodium hydroxide under normal pressure to keep the pH value of the reaction system at 13-14, and keep the reaction at 80-90℃ After 2.7 hours, acidify the system with 37% hydrochloric acid until the pH value of the system is 1.5, react at 50-60°C for 0.6h, filter again, and dry at 90-110°C to obtain 182.3g of 3, with a content of 99.0%. 5-Dichlorobenzoic acid.
[0045] Mix 100g of the obtained 3,5-dichlorobenzoic acid with 74.7g of thionyl chloride (content 99%), reflux at 70-80°C for 3.0h, and then recover chlorine at normal pressure and 75-82°C sulfoxide, and then distill 3,5-dichlorobenzoyl chloride at 185-195°C and -0.15MPa for the next reaction.
[0046] Take 52.8g of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com