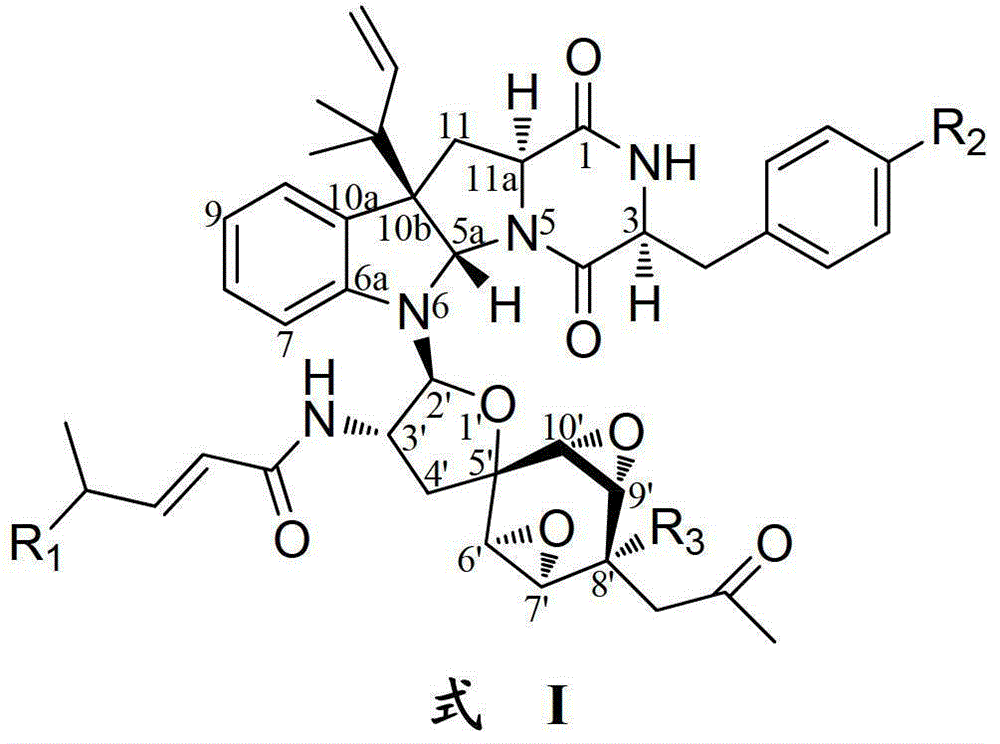
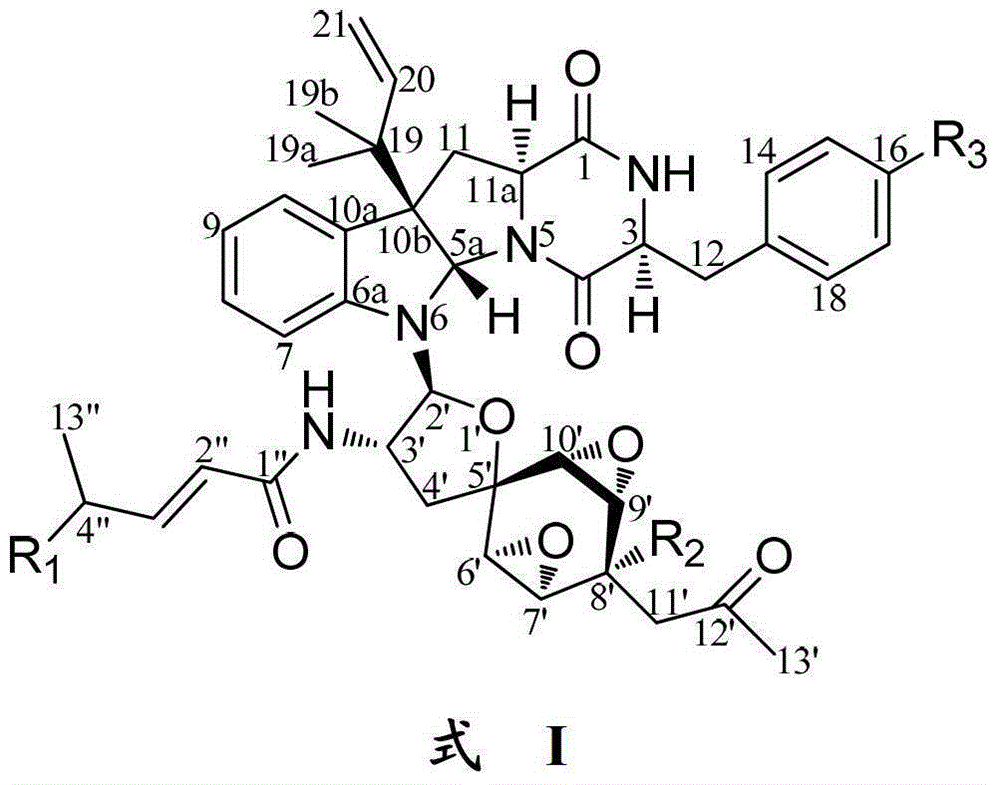
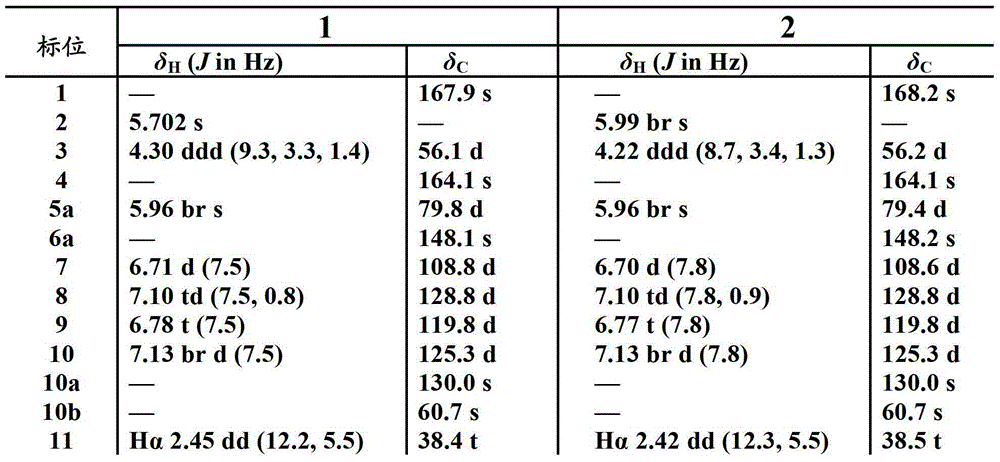
Indoline diketopiperazine spiro compound and its preparation method and use
A compound and straight-chain technology, applied in the field of preparation of spiro compounds, indolin diketopiperazine spiro compounds, and indolin diketopiperazine spiro compounds, and can solve the problem that there is no literature record, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Embodiment 1: Microbial fermentation culture and the preparation of compound 1 and compound 2
[0098] 1. Fermentation culture and extraction of fermented products
[0099] 1) Production strains
[0100] The toxin-producing bacteria used to ferment and produce compounds 1 and 2 in this example is Penicillium purpurogenum BD-1-3 (Penicillium purpurogenum BD-1-3) preserved in the General Microorganism Center of China Microbiological Culture Collection Management Committee, and the preservation number is 4284 (CGMCC No. 4283).
[0101] 2) Fermentation culture
[0102] From the PDA medium of Penicillium purpura BD-1-3 (composition: 2% glucose, 2% agar, 1.5% NaCl, prepared with 20% potato boiling liquid) test tube slant surface stored in the refrigerator at 4°C, use Scrape an appropriate amount of spores from the inoculation loop under sterile conditions, streak and inoculate them on a newly prepared PDA solid medium plate, and activate and cultivate them in a 28°C incub...
Embodiment 2
[0119] Example 2: Fermentation culture of Penicillium purpura 3-f-31 and separation and preparation of compound 1
[0120] 1. Fermentation culture and extraction of fermented products
[0121] 1) Production strains
[0122] The toxin-producing bacterium used to ferment and produce compound 1 in this example is the Penicillium purpurogenum 3-f-31 strain preserved in the General Microorganism Center of the China Microbiological Culture Collection Management Committee, and the preservation number is 7286 (CGMCCNo.7286 ).
[0123] 2) Fermentation culture
[0124] From the PDA medium (composition: 2% glucose, 2% agar, 1.5% NaCl, prepared with 20% potato boiling liquid) of the PDA medium of Penicillium purpura 3-f-31 stored in the refrigerator at 4°C, use Scrape the appropriate amount of spores from the inoculation loop under sterile conditions, streak and inoculate them on the newly prepared PDA solid medium plate, and cultivate them in a 28°C incubator for 3-5 days. When the...
Embodiment 3
[0134] Embodiment 3: Derivatization preparation of other compounds of formula I of the present invention 1a-1i and 2a-2r
[0135] 1) Derivatization preparation of compound 1a-1i of formula I of the present invention
[0136] Weigh about 10 mg of Compound 1 prepared in the above-mentioned Example 1 and Example 2, dissolve it with 0.5 ml of acetone, add 50 mg of anhydrous K 2 CO 2 Mix well, and add 50 μl methyl iodide dropwise under magnetic stirring at 60°C to carry out methylation reaction for 6 h. The reaction product was separated and purified by preparative silica gel thin-layer chromatography (chloroform-methanol volume ratio 93:7). In addition to recovering 5 mg of raw material compound 1, 1a (1.3 mg, positive ion ESI-MSm / z: 877[ M+H] + , negative ion ESI-MSm / z:875[M-H] - ).
[0137] Weigh about 10 mg of Compound 1 prepared in the above-mentioned Example 1 and Example 2, dissolve it with 0.5 ml of acetone, add 50 mg of anhydrous K 2 CO 2 Mix well, and add 50 μl d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com