Inorganic salt phase change cold storage material
A technology of phase change cold storage and inorganic salt, applied in the direction of heat exchange materials, chemical instruments and methods, etc., can solve the problems of high degree of supercooling, serious relationship, poor work performance, etc., and achieve low degree of supercooling and energy storage High density and good cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
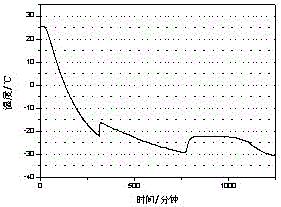
[0017] Pour 200mL of water into a 500mL beaker, add 56g of sodium chloride at a temperature of 25°C, stir until completely dissolved, then raise the temperature to 35°C, add 2.24g of sodium tetraborate to dissolve, add 1.12g of bentonite and stir for 10 minutes A phase change cold storage material is obtained.
[0018] Through the three-layer calorimeter test of w&a company, the phase change latent heat of the phase change cold storage material is 285J / g, and the supercooling degree is 0.9℃
[0019] The test result of this embodiment is as figure 2 shown.
Embodiment 2
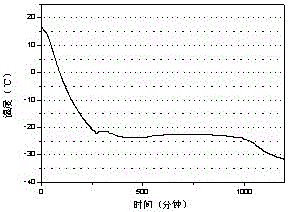
[0021] Pour 300mL of water into a 500mL beaker and add 79.8g of Mgcl at a temperature of 25°C 2 , stir until completely dissolved, then warm up to 35°C, add 4g of sodium tetraborate to dissolve, add 1.6g of bentonite and stir for 10 minutes, then add sodium alginate according to the standard of 5g of sodium alginate / 100mL solution and fully swell to obtain a phase change cold storage material.
[0022] Through the three-layer calorimeter test of w&a company, the phase change latent heat of the phase change cold storage material is 220J / g, and the supercooling degree is 2.5°C
[0023] The test result of this embodiment is as image 3 shown.
Embodiment 3
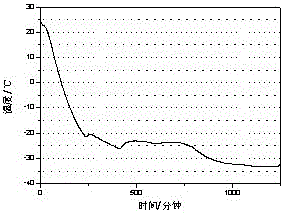
[0025] Pour 200mL of water into a 500mL beaker, add 44g of sodium nitrate at a temperature of 25°C, stir until completely dissolved, then raise the temperature to 35°C, add 1.32g of silica and 2.2g of bentonite and stir for 10 minutes to obtain a phase transition cold storage material.
[0026] Through the three-layer calorimeter test of w&a company, the phase change latent heat of the phase change cold storage material is 232J / g, and the supercooling degree is 0.8℃
[0027] The test result of this embodiment is as Figure 4 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition enthalpy | aaaaa | aaaaa |
| phase transition enthalpy | aaaaa | aaaaa |
| phase transition enthalpy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com