RelA cut and TLR7 active sequence modified locked nucleic acid deoxyribozyme for targeted therapy of tuberculosis and application thereof
A targeted therapy and deoxyribozyme technology, applied in gene therapy, DNA/RNA fragments, recombinant DNA technology, etc., can solve the problems of susceptibility to DNase degradation, insufficient half-life of 10-23 DNAzyme, single function, etc., to achieve strong Effect of specificity, good biosafety and development prospect, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: the design, synthesis of 3 locked nucleic acid deoxyribozymes of the present invention
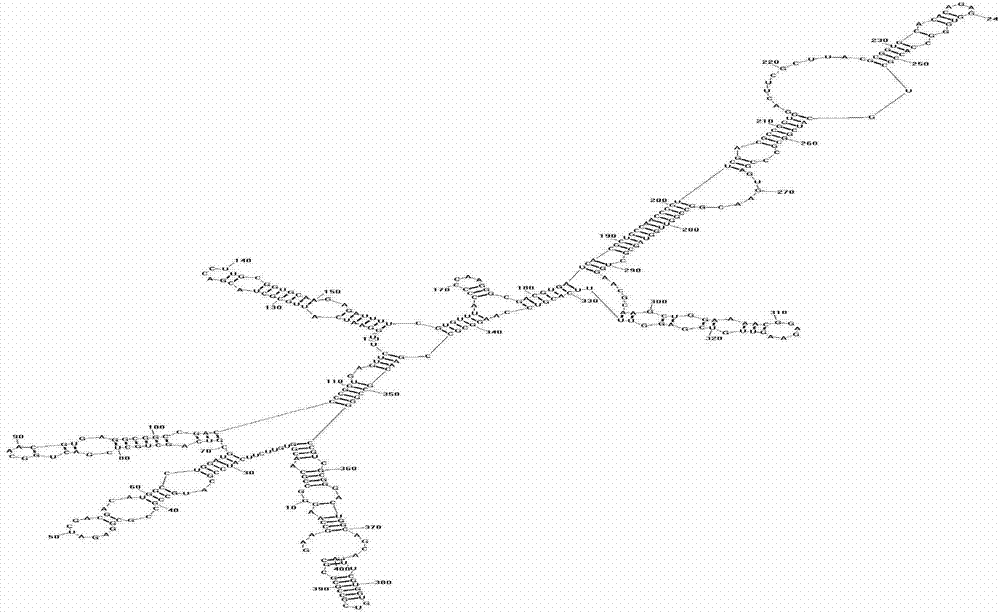
[0054] 1. Using locked nucleic acid as the synthetic raw material for the alternative part of DNAzyme, using the 10-23 DNAzyme structure as the basic framework, using computer software to simulate the secondary structure of RelA-mRNA ( figure 1 ), select a suitable target to be cut according to the simulated structure diagram and design a specific locked nucleic acid deoxyribozyme for the corresponding target. Perform performance analysis, screening and synthesis, hybridization and modification of RNA components composed of specific motifs. A hybrid modification was performed with the "U"-rich RNA sequence of the TLR7-activating RNA motif at the end of the 10-23 deoxyribozyme-specific recognition binding arm.
[0055] 2. According to the cleavage pattern of 10-23 DNAzyme, design a series of corresponding 10-23 locked nucleic acid DNAzyme sequences with different subst...
Embodiment 2
[0060] Example 2: Obtaining Mycobacterium tuberculosis RelA full-length mRNA with an in vitro transcription system
[0061] Mycobacterium tuberculosis H was extracted according to the instructions of the bacterial genome DNA extraction kit (Dalian TaKaRa Company) 37 Genomic DNA of Rv.
[0062] The upstream and downstream primers were designed according to the RelA mRNA coding sequence registered in GenBank, and synthesized by Beijing Sanbo Yuanzhi Biotechnology Co., Ltd.
[0063] Upstream: 5'-GGGATCCGATATCATGGCCGAGGACCAGC-3'
[0064] Downstream: 5'-GCGGCCGC AAGCTTCTACGCGGCCGAGGT-3'
[0065] Mycobacterium tuberculosis H 37 Genomic DNA of Rv was used as a template to carry out PCR reaction to obtain RelA gene.
[0066] After the PCR product was recovered, it was double-digested with endonuclease EcoRV / Hind III (purchased from Dalian TaKaRa Company, Shenzhen Jingmei Company, British NEB Company, and American Promega Company) and cloned into the vector pET-32a(+) (purchased f...
Embodiment 3
[0069] Embodiment 3: Observation of the cutting effect of each locked nucleic acid deoxyribozyme on Rel A mRNA extracellularly
[0070] Add the corresponding reagents (μL) to the 7 reaction tubes according to the table below:
[0071]
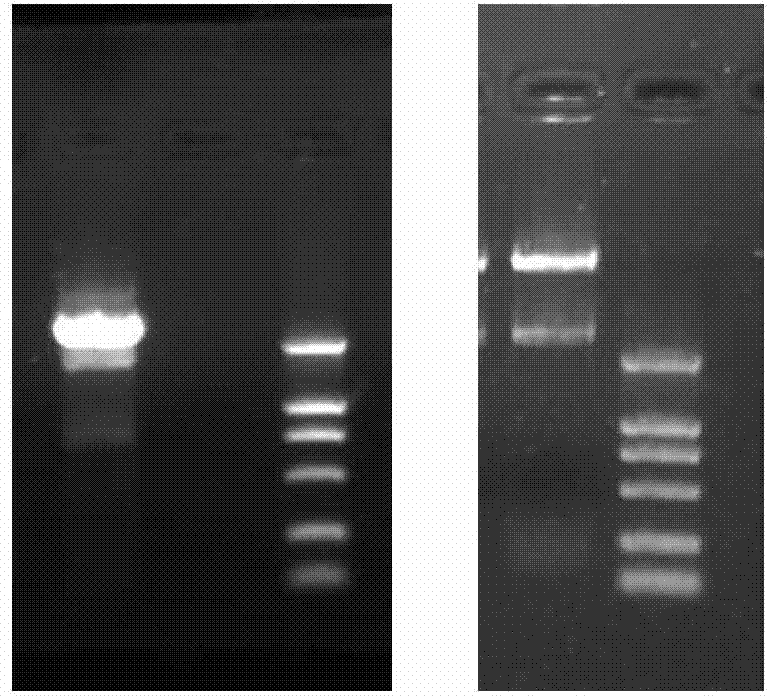
[0072] Each reaction tube was incubated at 37°C for 60 min, then taken out, and separated by electrophoresis on a 3.5% denaturing polyacrylamide gel. Then use the nucleic acid silver staining kit (purchased from Beijing Dingguo Company, and can also be purchased from Beijing Zhongshan Company and Shanghai Shenggong Company) for staining, and then use the gel imaging system to collect images, measure the optical density of each band, and calculate cut percentage. Cleavage percentage (%) = total optical density value of cleaved product bands / (optical density value of uncleaved substrate bands + total optical density value of cleaved product bands) × 100%.
[0073] Results: The cleavage systems of LDZ4 and LDZ5 had obvious specific cleavage p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com