Method for determining content of sulfite in medicinal materials by headspace gas chromatography-mass spectrometry
A headspace gas chromatography and sulfite technology, which is applied in the field of detection of sulfite content in medicinal materials, can solve the problems of inaccurate detection results, prolonged reaction time, and high temperature of the headspace bottle, so as to overcome the reaction time and equilibrium time. Shortened, high-accuracy effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
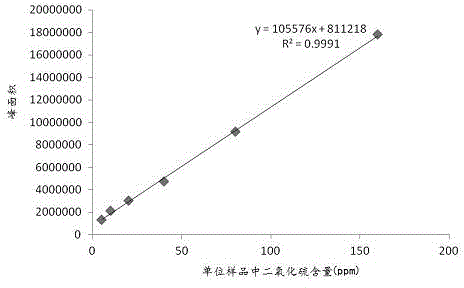
[0035] (1) Accurately weigh 0.50 g of sodium sulfite solid powder, add it into a 100ml volumetric flask for ultrasonic dissolution, and constant volume to obtain a standard stock solution; respectively take 100, 200, 400, 800, 1600 and 3200 μl of the standard stock solution, Dilute to 25 ml volumetric flasks numbered A1, A2, A3, A4, A5 and A6 in turn to obtain 20, 40, 80, 160, 320 and 640 μg / ml sodium sulfite standard solutions;
[0036] (2) Add 2 g of pulverized Lycium sulfite-free granules, 400 μl of 20 μg / ml sodium sulfite standard solution to the headspace vial numbered B1, and then add 5 ml of 10 % sulfuric acid solution, quickly seal the bottle cap, shake up to obtain the standard test solution C1, the sulfite content of the unit medicinal material in the standard test solution C1 is 5 ppm after conversion;
[0037] According to the method of step (2), add 2 g of pulverized Lycium barbarum granules without sulfite to the headspace vials numbered B2, B3, B4, B5, and B6, a...
Embodiment 2
[0042] (1) Accurately weigh 0.50 g of sodium sulfite solid powder, add it into a 100ml volumetric flask for ultrasonic dissolution, and constant volume to obtain a standard stock solution; respectively take 100, 200, 400, 800, 1600 and 3200 μl of the standard stock solution, Dilute to 25 ml volumetric flasks numbered A1, A2, A3, A4, A5 and A6 in turn to obtain 20, 40, 80, 160, 320 and 640 μg / ml sodium sulfite standard solutions;
[0043] (2) Add 2 g of pulverized sulfite-free angelica granules, 400 μl of 20 μg / ml sodium sulfite standard solution, and then add 5 ml of 25% sulfuric acid solution, quickly seal the bottle cap, shake well to obtain the standard test solution C1, the sulfite content of the unit medicinal material in the standard test solution C1 is 5 ppm after conversion;
[0044] According to the method of step (2), add 2 g of crushed sulfite-free Angelica granules into the headspace vials numbered B2, B3, B4, B5, and B6 respectively, and add them to different head...
Embodiment 3
[0049] (1) Accurately weigh 0.50 g of sodium sulfite solid powder, add it into a 100ml volumetric flask for ultrasonic dissolution, and constant volume to obtain a standard stock solution; respectively take 100, 200, 400, 800, 1600 and 3200 μl of the standard stock solution, Dilute to 25 ml volumetric flasks numbered A1, A2, A3, A4, A5 and A6 in turn to obtain standard solutions of 20, 40, 80, 160, 320 and 640 μg / ml;
[0050] (2) Add 2 g of pulverized sulfite-free Codonopsis granules to the headspace vials numbered B1, B2, B3, B4, B5, and B6, and add them to different headspace vials in turn. 400 μl of sodium sulfite standard solutions with concentrations of 20, 40, 80, 160, 320 and 640 μg / ml respectively, and finally add 8 ml of sulfuric acid solution with a mass concentration of 30% to different numbered headspace vials, and quickly seal the vials Cover, shake well, and obtain standard test solutions C1, C2, C3, C4, C5, and C6 in sequence. After conversion, the sulfite conte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com