A kind of non-precious metal electrocatalyst for fuel cell and preparation method thereof
An electrocatalyst, non-precious metal technology, applied in the field of non-precious metal fuel cell oxygen reduction electrocatalyst and its preparation, can solve the problems of ambiguous research on electrocatalytic oxygen reduction performance active sites, complex catalyst preparation process, harsh reaction conditions, etc. Good stability, good electrocatalytic activity, and strong controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
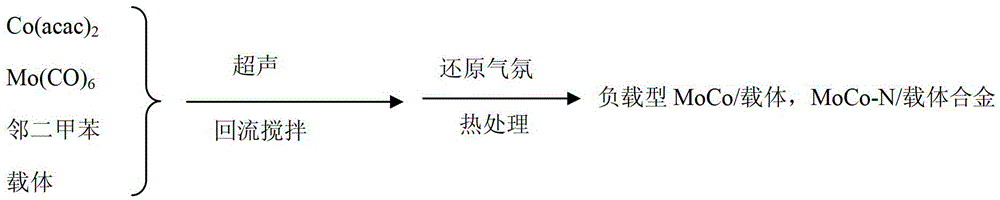
[0032] The synthesis process of the electrocatalyst used in the PEMFC (proton-exchangemembranefuelcells proton-exchange membrane fuel cells) of the present embodiment can be found in figure 1 ,Specifically:
[0033] 0.2mmol molybdenum hexacarbonyl (Mo(CO) 6 ) with 0.2mmol cobalt acetylacetonate (Co(acac) 2 ) and 66mg of carrier XC-72 were added into a 150mL o-xylene solution (the volume of ortho-xylene is not limited, as long as the precursor and the carrier are uniformly dispersed), and dispersed evenly by ultrasound. Under the condition of using o-xylene as solvent and reducing agent, heat up and stir to reflux ("reflux" is to ensure that under the boiling point conditions of o-xylene and molybdenum hexacarbonyl, on the one hand, molybdenum hexacarbonyl can reach the cracking condition, on the other hand ortho-xylene acts as a solvent and a reducing agent), the temperature was controlled at 145° C., and the loaded precursor was obtained after 4.5 h of reaction, filtered, w...
Embodiment 2
[0037] Synthesis process see figure 1 ,Specifically:
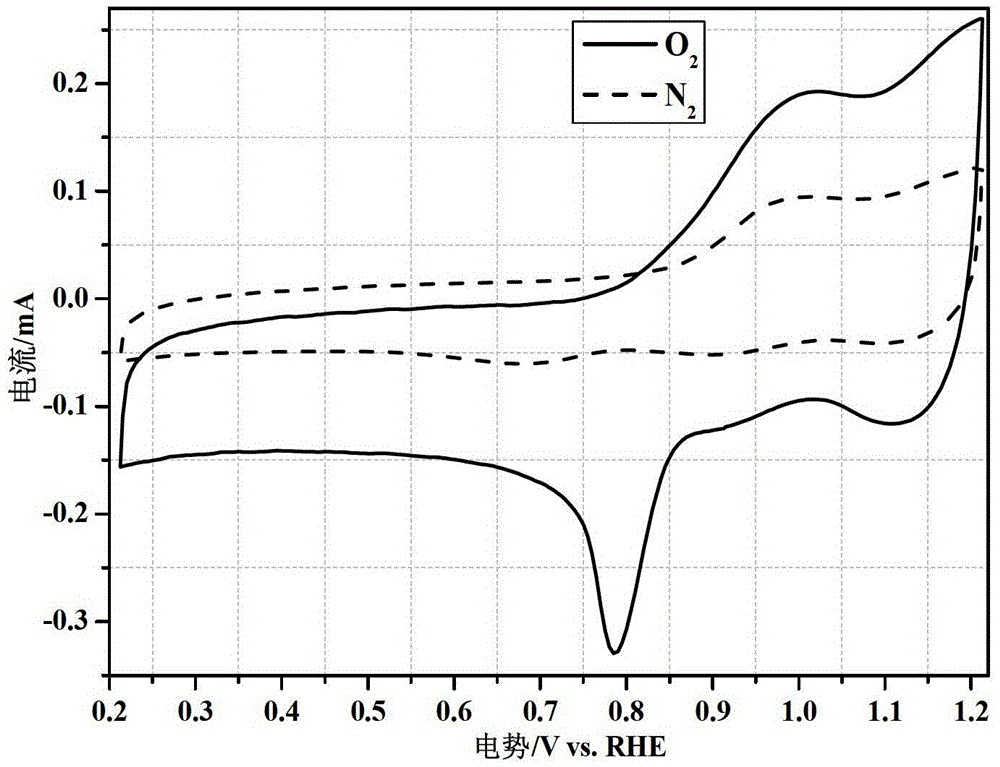
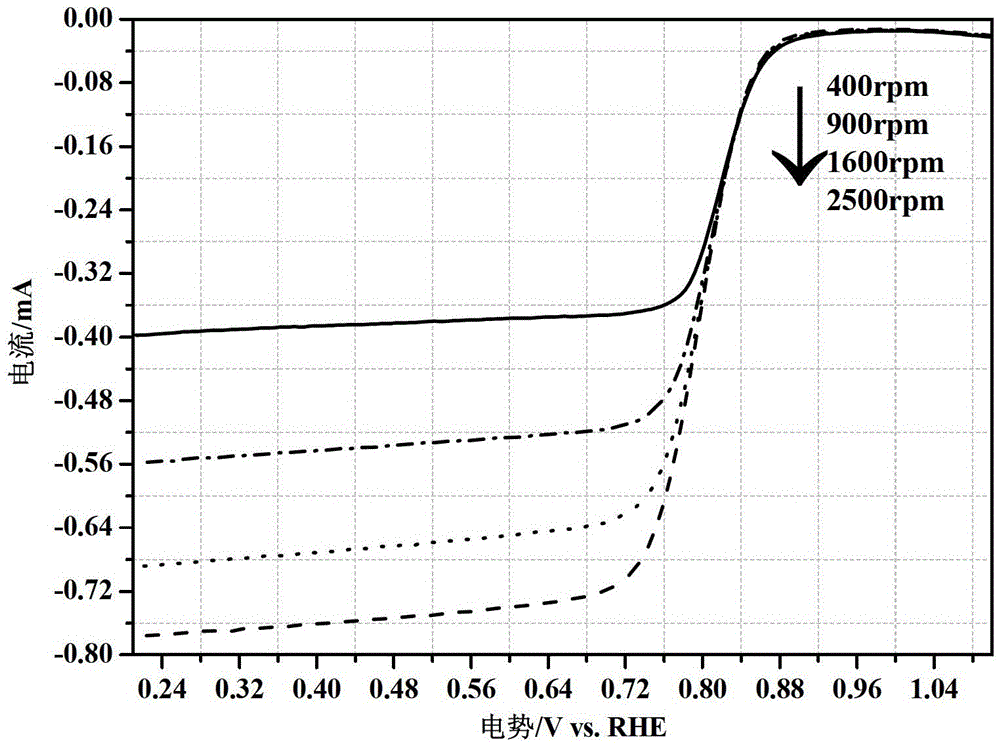
[0038] 0.2mmol molybdenum hexacarbonyl (Mo(CO) 6 ) with 0.2mmol cobalt acetylacetonate (Co(acac) 2 ) and 66mg of carrier XC-72 were added to a 150mL o-xylene solution, and under the condition of using o-xylene as a solvent and a reducing agent, the temperature was raised and stirred to reflux, and the temperature was controlled at 140°C, 145°C, and 155°C respectively. After 4.5 hours, the loaded precursor was obtained after filtering, washing and drying. The X-ray diffraction pattern of the resulting precursor is shown in Figure 7 As shown, the three curves a, b, and c respectively correspond to reflux temperatures of 155°C, 145°C, and 140°C.
[0039] After grinding the precursors obtained at different reflux temperatures, the supported MoCo-N / carrier alloy was obtained after heat treatment at a low temperature of 550 °C for 3 h with ammonia gas as a reducing atmosphere. Its X-ray diffraction picture is as Figure 8...
Embodiment 3
[0041] Synthesis process see figure 1 ,Specifically:
[0042] 0.2mmol molybdenum hexacarbonyl (Mo(CO) 6 ) with 0.2mmol cobalt acetylacetonate (Co(acac) 2 ) and 66mg of carrier XC-72 were added to a 150mL o-xylene solution, and under the condition of using o-xylene as a solvent and a reducing agent, the temperature was raised and stirred to reflux, the temperature was controlled at 145°C, and the reaction time distribution was 2.5h, 3.5 h, 4.5h, after filtering, washing and drying, the loaded precursor was obtained. Figure 9 X-ray diffraction patterns of the prepared electrocatalyst MoCo / support for PEMFC at different reflux times, where the three curves a, b, and c correspond to the reflux times of 3.5h, 2.5h, and 4.5h, respectively.
[0043] After grinding the precursors obtained under different reflux times, the supported MoCo-N / carrier alloy was obtained after heat treatment at a low temperature of 550 °C for 3 h with ammonia gas as a reducing atmosphere. Its X-ray dif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com