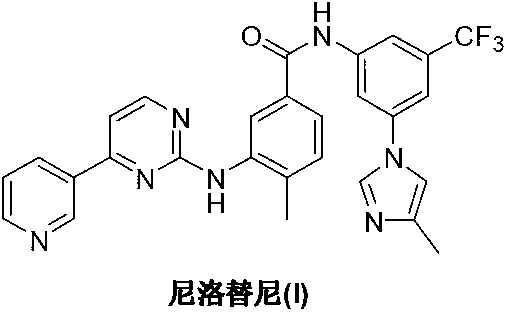
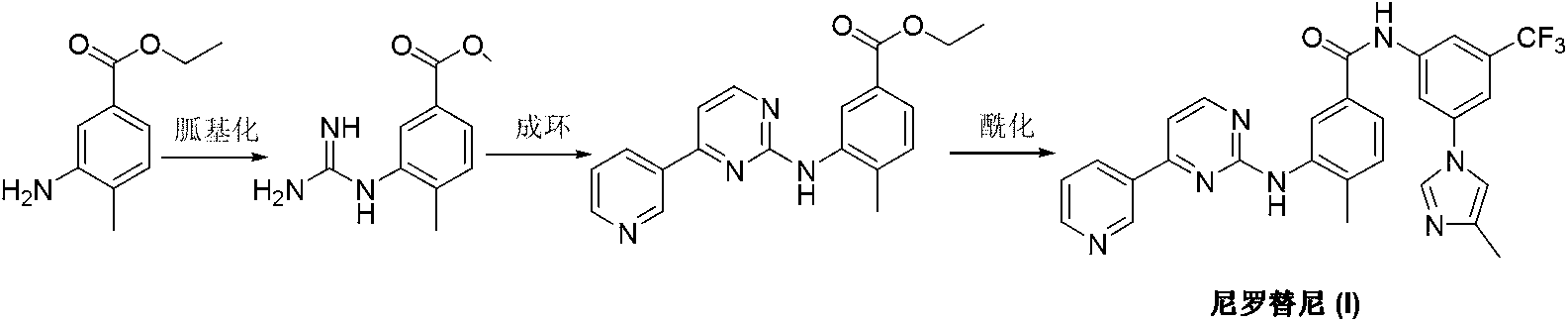
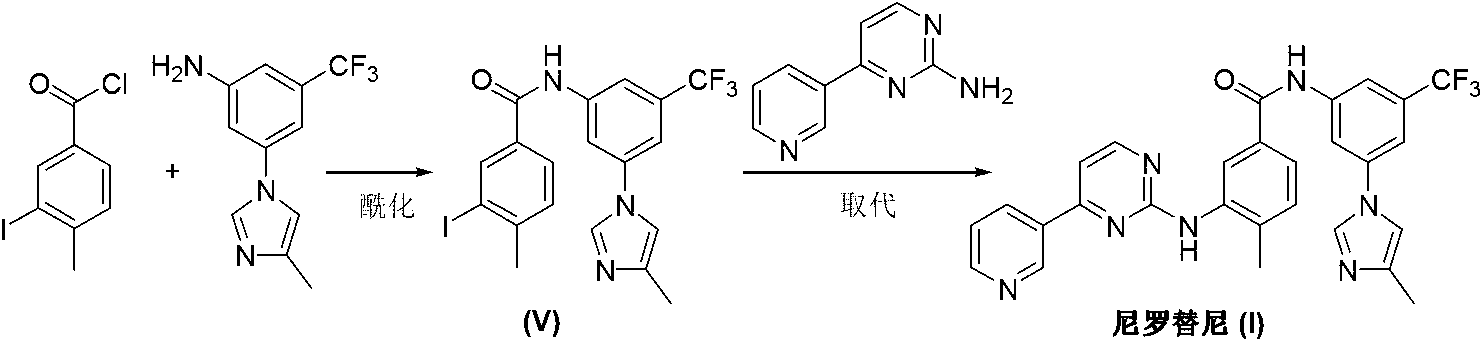
Preparation method of nilotinib
A nilotinib and condensation reaction technology, which is applied in the field of nilotinib preparation, can solve the problems of rare raw materials, high cost, and long steps, and achieve the effects of controllable production, improved product quality, and promoted development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Under nitrogen protection, 4-(3-pyridyl)-2-pyrimidinone (II) (1.73g, 10mmol), benzotriazol-1-yloxytris(dimethylamino) Phosphonium hexafluorophosphate (BOP) (6.63 g, 15 mmol) and acetonitrile 50 mL. Under stirring, 1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU) (2.28 g, 15 mmol) was added dropwise, and the reaction was carried out at room temperature for 12 hours. The temperature was raised to 70° C., methyl 3-amino-4-methylbenzoate (III) (2.15 g, 13 mmol) and potassium tert-butoxide (1.65 g, 15 mmol) were added, and the reaction was continued for 12 hours. About half of the volume of the solvent was distilled off under reduced pressure, 30 mL of 2M sodium hydroxide was added, and the mixture was reacted at 80°C for 6 hours. Extracted 3 times with 120 mL of ethyl acetate, separated the organic phase, dried and concentrated under reduced pressure. The residue was dissolved in 100 mL THF, 5-(4-methyl-1H-imidazol-1-yl)-3-trifluoromethylaniline (IV) (2.89 g, 12 mmol) and 1,8-di...
Embodiment 2
[0027] Under nitrogen protection, 4-(3-pyridyl)-2-pyrimidinone (II) (1.73g, 10mmol), benzotriazol-1-yloxytris(dimethylamino) Phosphonium hexafluorophosphate (BOP) (6.63 g, 15 mmol) and acetonitrile 50 mL. Under stirring, 1,5-diazabicyclo[4.3.0]-non-5-ene (DBN) (1.86 g, 15 mmol) was added dropwise, and the reaction was carried out at room temperature for 12 hours. The temperature was raised to 70° C., methyl 3-amino-4-methylbenzoate (III) (2.15 g, 13 mmol) and potassium tert-butoxide (1.65 g, 15 mmol) were added, and the reaction was continued for 12 hours. About half of the volume of the solvent was distilled off under reduced pressure, 30 mL of 2M sodium hydroxide was added, and the mixture was reacted at 80°C for 6 hours. Extracted 3 times with 120 mL of ethyl acetate, separated the organic phase, dried and concentrated under reduced pressure. The residue was dissolved in 100 mL THF, and 5-(4-methyl-1H-imidazol-1-yl)-3-trifluoromethylaniline (IV) (2.89 g, 12 mmol) and 1,5-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com