Ketorolac implant and preparation method thereof
A technology of ketorolac and implants, which is applied in the field of medicine and can solve difficult problems such as realization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
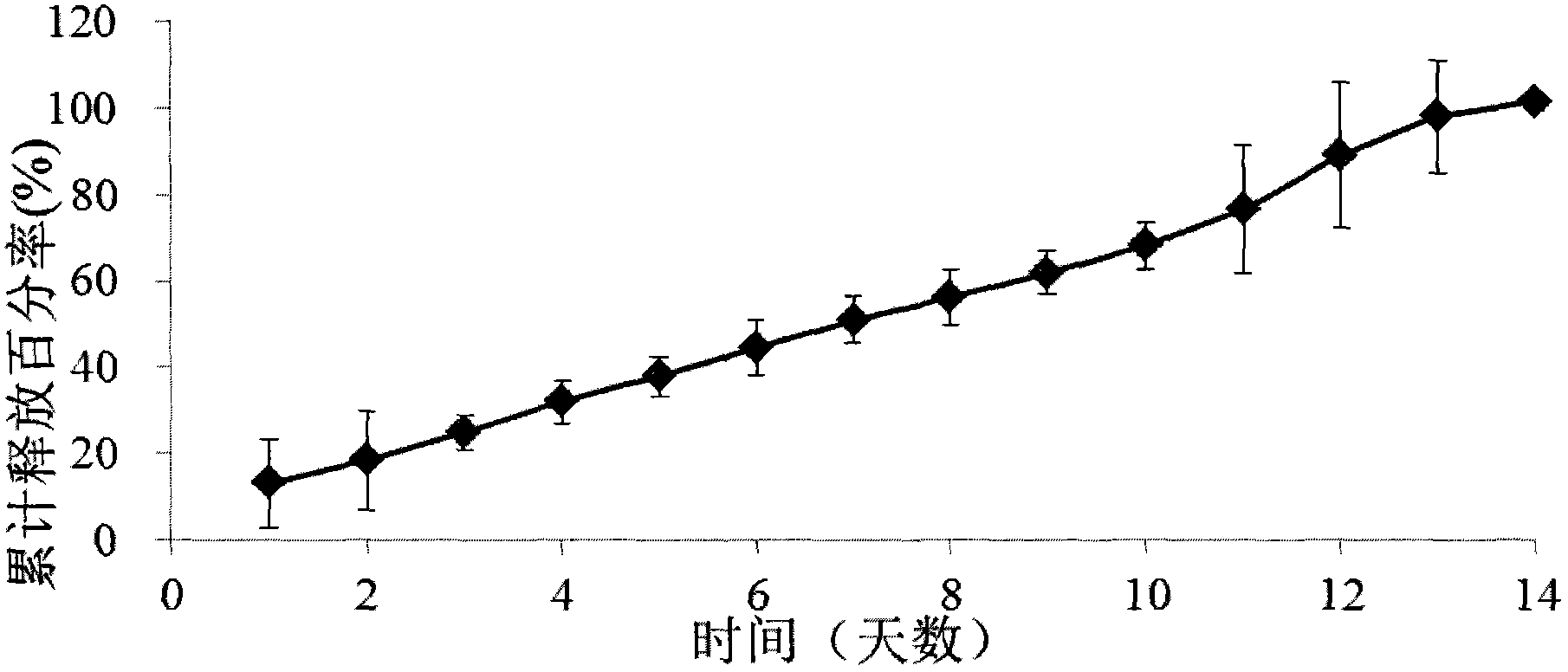
[0067] Embodiment 1: Ketorolac implant (drug release for 2 days)
[0068] 1) Preparation of ketorolac implant (drug release for 2 days)
[0069] By weight percentage, 30% of ketorolac tromethamine and 20% of poly D, L-lactic acid (carboxyl-terminated, molecular weight of 18kDa to 28kDa) and 40% of poly D, L-lactic acid-CO-ethanol Acid (carboxyl-terminated, lactic acid and glycolic acid ratio 50:50, molecular weight range 7kDa to 17kDa) and 10% polyethylene glycol (PEG4000) mixed, put into Turbula three-dimensional mixer (T2F type, WAB machinery company) and 4 stainless steel balls were shaken twice, each time for 15 minutes. The mixture was then put into a HAAKE miniature twin-screw extruder (MiniLab type, Thermo Scientific Company), the temperature of the extruder was set at 66° C., and the speed was set at 25 revolutions per minute. The diameter of the extruded rod is 0.5mm. The small stick-shaped implant was cut with a razor blade into small test samples with a length of...
Embodiment 2
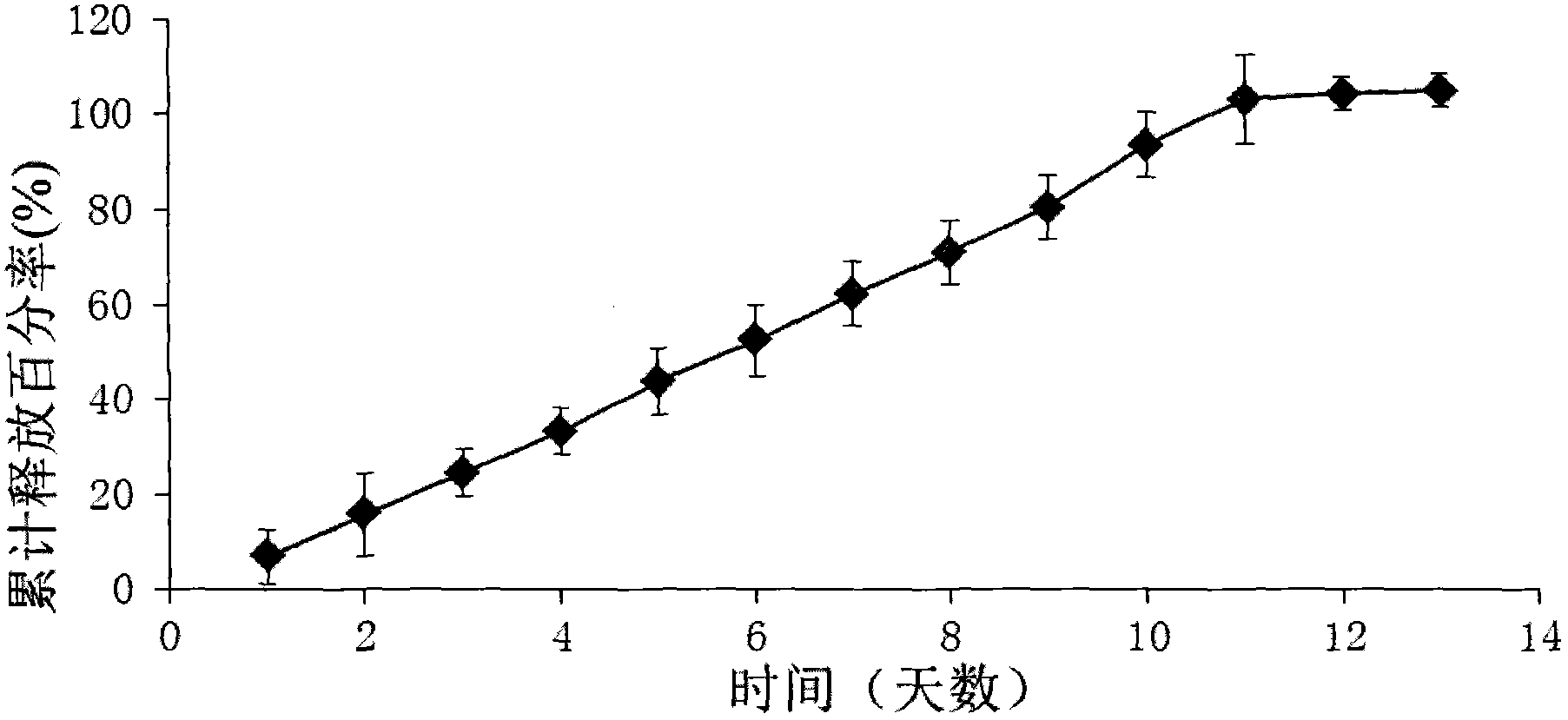
[0074] Embodiment 2: Ketorolac implant (drug release for 5 days)
[0075] 1) Preparation of ketorolac implant (drug release for 5 days)
[0076] In terms of weight percentage, 50% of ketorolac tromethamine and 30% of poly D, L-lactic acid (carboxyl-terminated, with a molecular weight of 18kDa to 28kDa) and 20% of poly D, L-lactic acid-CO-ethanol Acid (carboxyl-terminated, lactic acid to glycolic acid ratio 50:50, molecular weight range 7kDa to 17kDa) mixed, put into Turbula three-dimensional mixer (T2F type, WAB Machinery Company) and 4 stainless steel balls to shake twice, each time 15 minute. Then the mixture was put into a HAAKE miniature twin-screw extruder (MiniLab type, Thermo Scientific Company), the temperature of the extruder was set at 78° C., and the speed was set at 25 revolutions per minute. The diameter of the extruded rod is 0.5mm. The small rod-shaped implants were cut with a blade into small pieces with a length of 2 mm.
[0077] 2) In vitro release test: ...
Embodiment 3
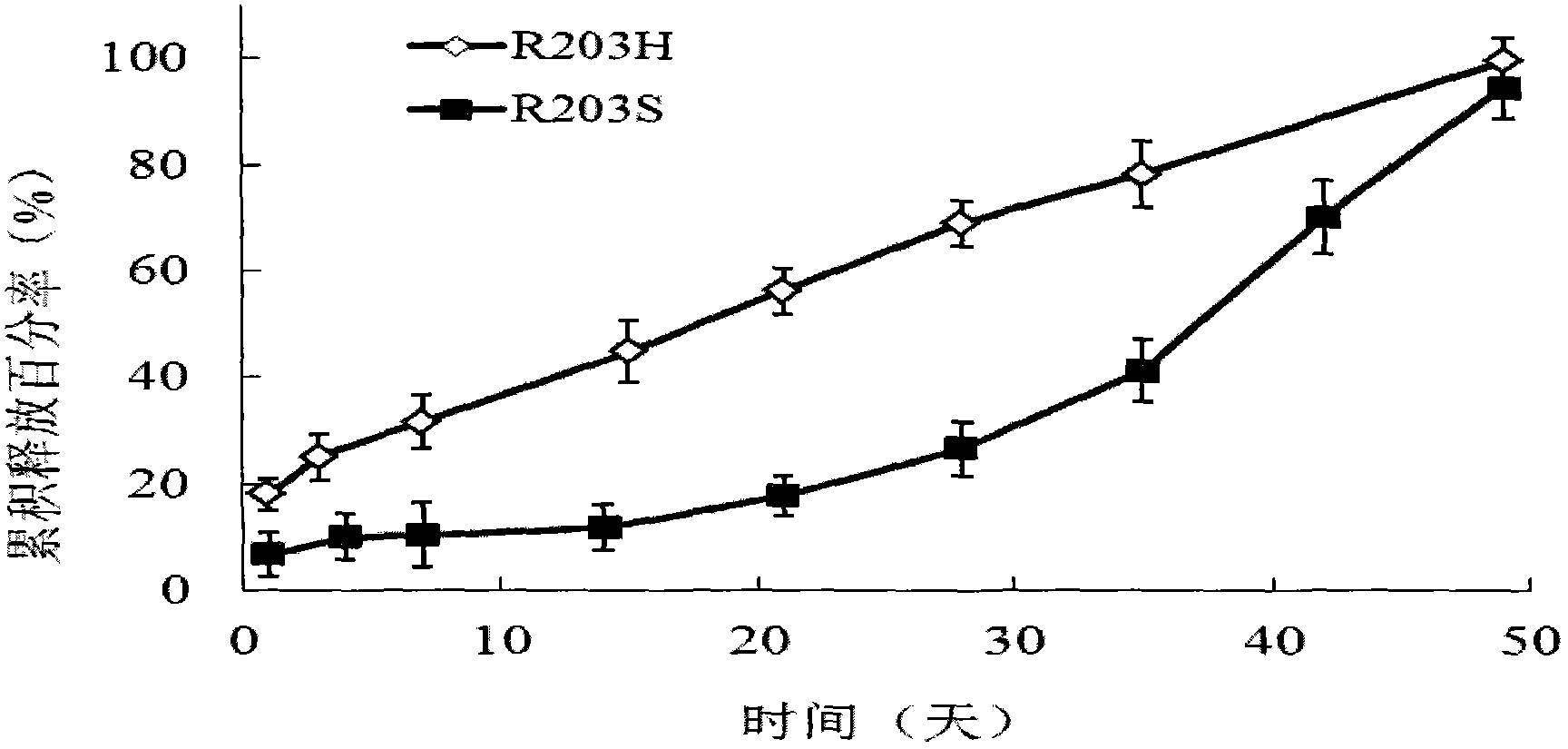
[0080] Embodiment 3: Ketorolac implant (11 days of drug release)
[0081] 1) Preparation of ketorolac implant (drug release for 11 days)
[0082] Five groups of ketorolac implants with drug release for 11 days were prepared by using different contents of ketorolac trometamol and rate modifiers, as well as different molecular weights and contents of carboxyl-terminated PLA and carboxyl-terminated PLGA, as shown in the following table 2 shows:
[0083] Table 2: Ketorolac Implants Released for 11 Days
[0084]
[0085] The preparation method of implant B is as follows:
[0086]By weight percentage, 40% of ketorolac tromethamine and 25% of poly D, L-lactic acid (carboxyl-terminated, molecular weight 10kDa to 18kDa) and 35% of poly D, L-lactic acid-CO-ethanol Acid (carboxyl-terminated, lactic acid to glycolic acid ratio 50:50, molecular weight range 7kDa to 17kDa) mixed, put into Turbula three-dimensional mixer (T2F type, WAB Machinery Company) and 4 stainless steel balls to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com