Preparation and application of anti-tumor activator nanostructure lipid carrier
A nano-structured lipid and surfactant technology, applied in the field of biomedicine, can solve the problems of limitation, poor water solubility, and unstable metabolism, and achieve good anti-tumor effects and improve poor solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
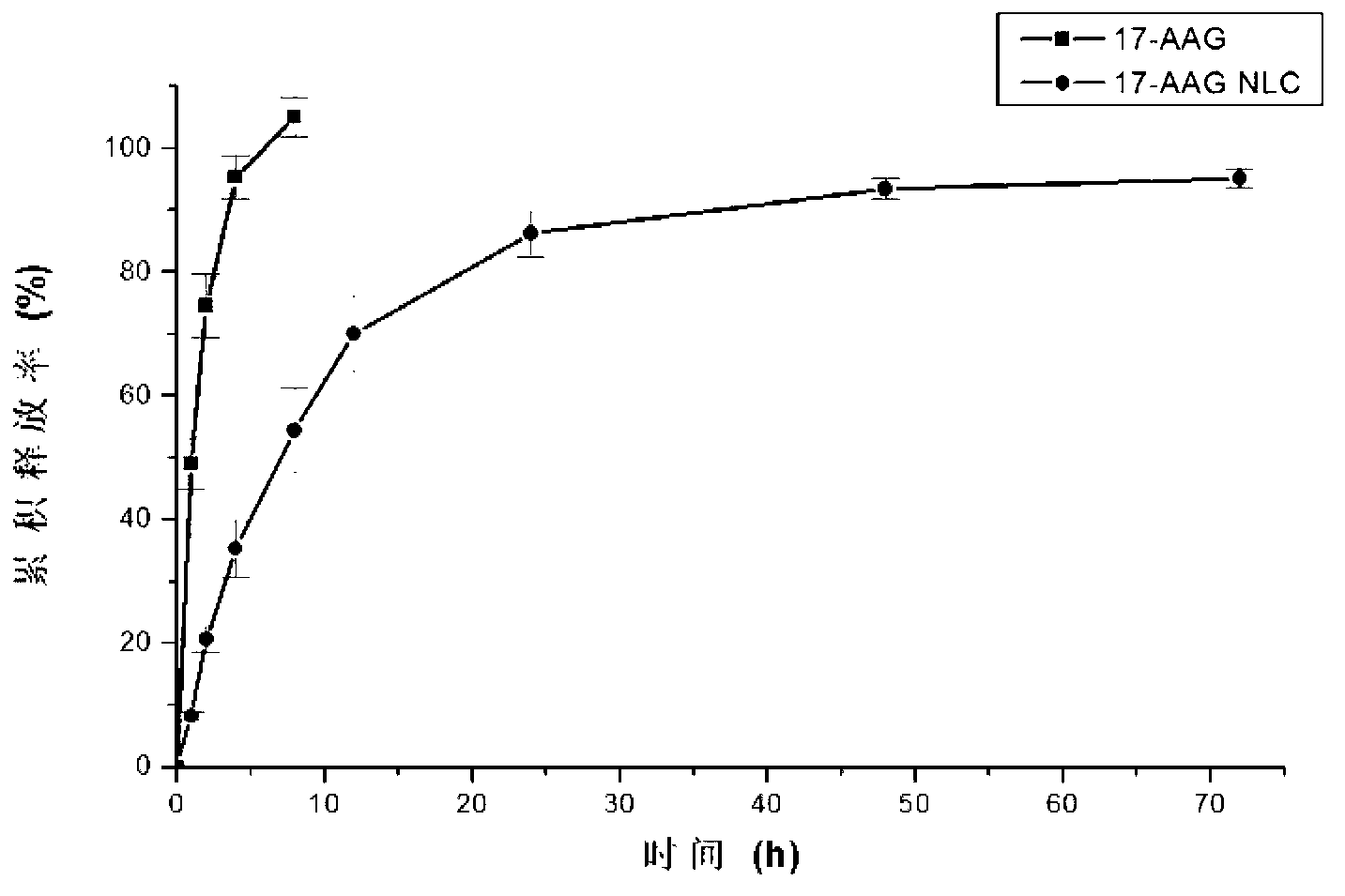
Image
Examples
Embodiment 1
[0075] Example 1: Preparation of Blank Nanostructured Lipid Carrier
[0076] Weigh 0.333g glyceryl monostearate and 0.666g caprylic acid / capric triglyceride in a 100ml eggplant-shaped bottle with an analytical balance, heat in a water bath, and keep the temperature at 60°C. Weigh 0.4g Tween 80 and stir to disperse in PBS buffer (PH7.4) (preparation method: 8gNaCl+0.2gKCl+2.94gNa 2 HPO 4 12H 2 O+0.2g KH 2 PO 4 , distilled water to 1000ml). After the solid-liquid phase lipid is melted, under stirring, the PBS buffer solution dispersed with Tween 80 is added dropwise into the molten solid-liquid lipid, and the stirring is continued to obtain a pre-emulsion, and the volume is constant. The pre-emulsion is subjected to probe ultrasonication, and the probe is ultrasonically cooled to room temperature to form a nanostructured lipid carrier water dispersion system.
Embodiment 2
[0077]Example 2: Preparation of 17-propenylamino-17-desmethoxygeldanamycin nanostructured lipid carrier
[0078] Weigh 0.333g of glyceryl monostearate and 0.666g of caprylic / capric triglyceride in a 100ml eggplant-shaped bottle with an analytical balance, heat in a water bath, and keep the temperature at 60°C. After the solid-liquid phase lipids are melted, stir 1 ml of 17-AAG ethanol solution with a concentration of 2 mg / ml was added dropwise. Weigh 0.4g Tween 80 and stir to disperse in PBS buffer (PH7.4) (preparation method: 8gNaCl+0.2gKCl+2.94gNa 2 HPO 4 12H 2 O+0.2gKH 2 PO 4 , distilled water to 1000ml). Under stirring, the PBS buffer solution that will be dispersed with Tween 80 is added dropwise in the solid-liquid lipid of melting, continues stirring to obtain pre-emulsion, constant volume. The pre-emulsion is subjected to probe ultrasonication, and the probe is ultrasonically cooled to room temperature to form a nanostructured lipid carrier water dispersion syste...
Embodiment 3
[0080] Blank nanostructured lipid carrier, the ratio of each component is as follows:
[0081] The sum of solid phase lipid and liquid phase lipid is 5% (W / V),
[0082] The ratio of solid phase lipids to liquid phase lipids, the mass ratio is: 1:0.5,
[0083] The content of surfactant is 1% (W / V),
[0084] The rest are solvents for injection.
[0085] Among them, the ratio of solid phase lipid and liquid phase lipid is 1:0.5. Wherein the solid phase lipid is preferably glyceryl monostearate, and the liquid phase lipid is caprylic acid / capric triglyceride.
[0086] Wherein said surfactant is preferably Tween 80.
[0087] Wherein the solvent for injection is originally selected from: water for injection, physiological saline, and isotonic PBS buffer solution of pH 7.4.
[0088] The preparation method of the blank nanostructured lipid carrier of the present invention is as follows: Weigh the solid-phase lipid and liquid-phase lipid, mix the above-mentioned components uniform...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com