Polyether sulphone material containing phenoxy aliphatic chain boric acid ester side chain and preparation method of material
A technology containing phenoxy fats and phenoxy fats, applied in the field of polyarylethersulfone polymer materials containing aliphatic chain borate phenyl side chains and its preparation, can solve solvent sensitivity, limited application, intolerance, etc. problems, achieve good thermal stability, simple and easy preparation method, and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
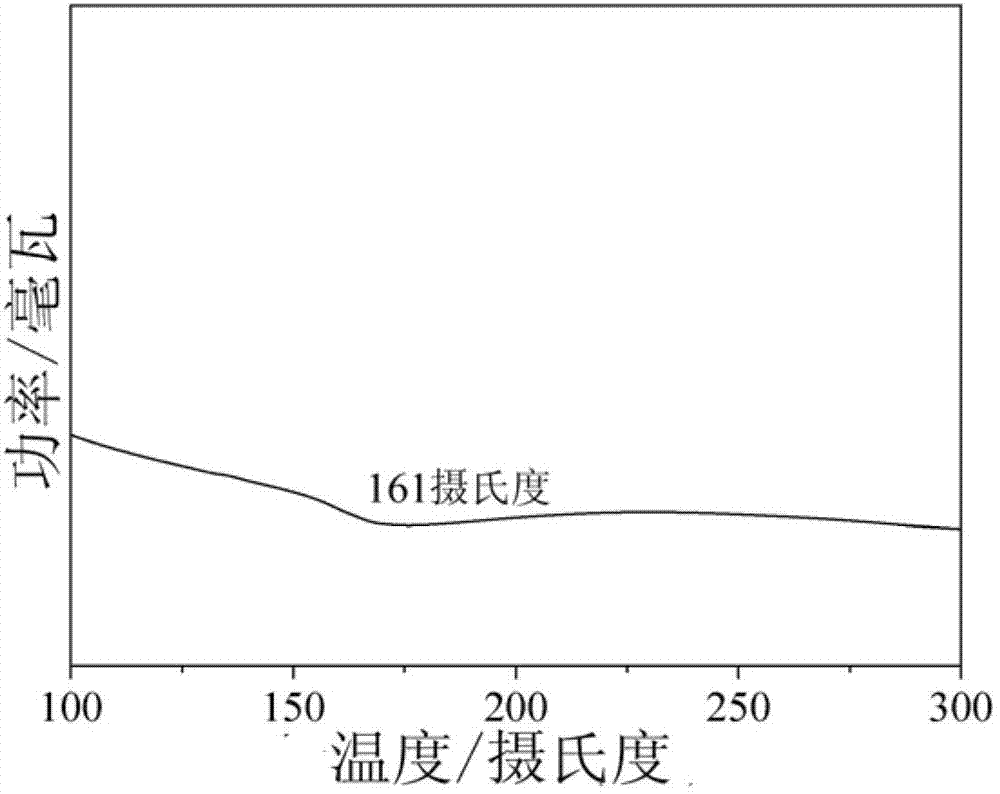
Image
Examples
Embodiment 1
[0038] Example 1: Preparation of polyaryl ether sulfone containing pendant methoxybenzene groups (1)
[0039] 10.0812g (0.05mol) 2-(3`-methoxyphenyl)-1,4-hydroquinone monomer, 12.7125g (0.05mol) 4,4`-difluorodiphenyl sulfone and 7.6005g Put anhydrous potassium carbonate, 75ml sulfolane, 25ml dehydrating agent (toluene or xylene) into a three-necked flask equipped with nitrogen through-holes, mechanical stirring and a water container, pour nitrogen through, start stirring, and heat to the reflux of the azeotropic dehydrating agent. React for 1 to 3 hours, remove the azeotropic dehydrating agent, and increase the temperature to 140-180°C to continue the reaction for 7-10 hours; then, the obtained polymer solution is precipitated in deionized water, crushed, washed, and dried to obtain an intermediate Pendant methoxybenzene polyaryl ether sulfone polymer.
[0040] The molecular formula is as follows:
[0041]
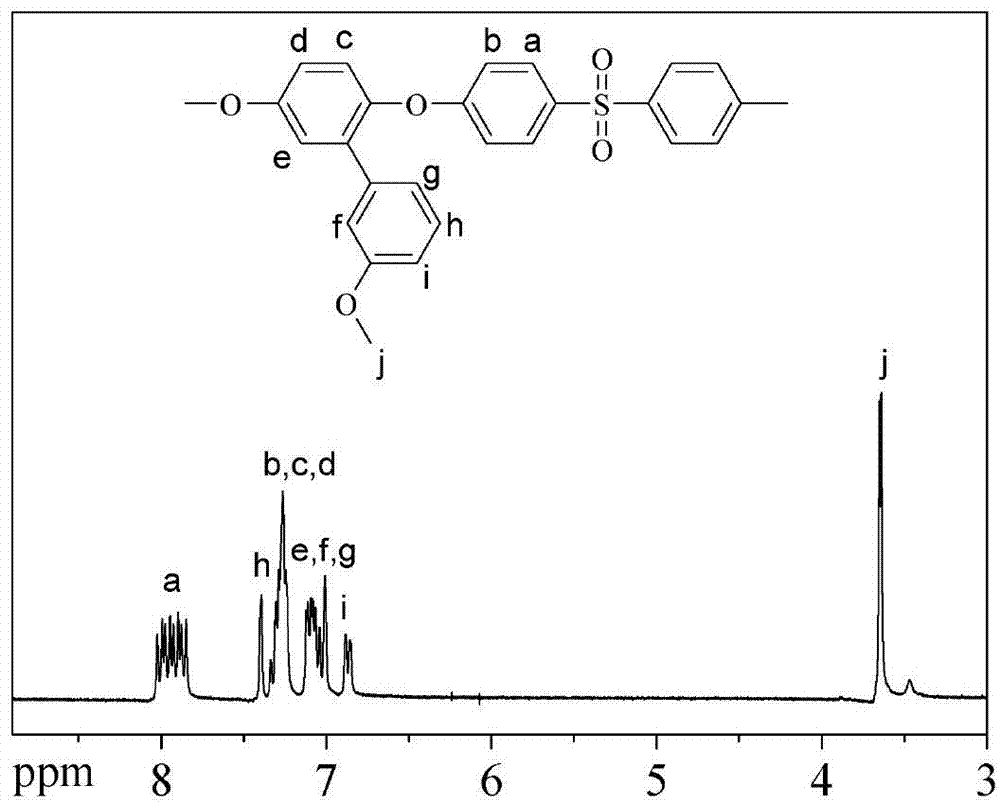
[0042] image 3 The hydrogen nuclear magnetic spectrum of the polyaryl eth...
Embodiment 2
[0045] Example 2: Preparation of polyaryl ether sulfone containing pendant hydroxyl benzene groups (1)
[0046] First add 322ml of pyridine to a three-necked flask with a thermometer, nitrogen through holes, mechanical stirring, and a constant pressure dropping funnel. Add 336ml of concentrated hydrochloric acid to the dropping funnel. Add the concentrated hydrochloric acid dropwise to the pyridine while stirring. After that, remove the constant pressure dropping funnel, install the distillation head, vacuum tail tube, single-neck flask, rubber tube, blow nitrogen and turn on the heating, slowly distill the water out, and stop heating when the temperature reaches 200~215°C. After room temperature, remove the distillation head, vacuum end adapter, and single-necked flask, add 5g of polyaryl ether sulfone polymer containing m-methoxyphenyl pendant groups into the three-necked flask, install the spherical condenser, turn on the heating, and increase the temperature to 160~ Reacting ...
Embodiment 3
[0052] Example 3: Preparation of polyaryl ether sulfone containing pendant allyloxybenzene groups (1)
[0053] Put 2.0823g of meta-hydroxy-containing polyaryl ether sulfone into a three-necked flask with thermometer, nitrogen through hole, mechanical stirring, and water container, then add 0.4491g anhydrous potassium carbonate, 10ml N,N-dimethylacetamide, 5ml Toluene, nitrogen gas, start stirring, heat up to the reflux of the azeotropic dehydrating agent, react for 1 to 3 hours, remove the azeotropic dehydrating agent, reduce the temperature to 30-50°C, add 2.2ml allyl bromide, install a spherical condenser, The temperature is raised to 100-120°C and the reaction is continued for 6-10 hours; then, the obtained polymer solution is precipitated in deionized water, crushed, washed, and dried to obtain polyaryl ether sulfone containing m-phenoxy allyl side groups polymer.
[0054] The molecular formula is as follows:
[0055]
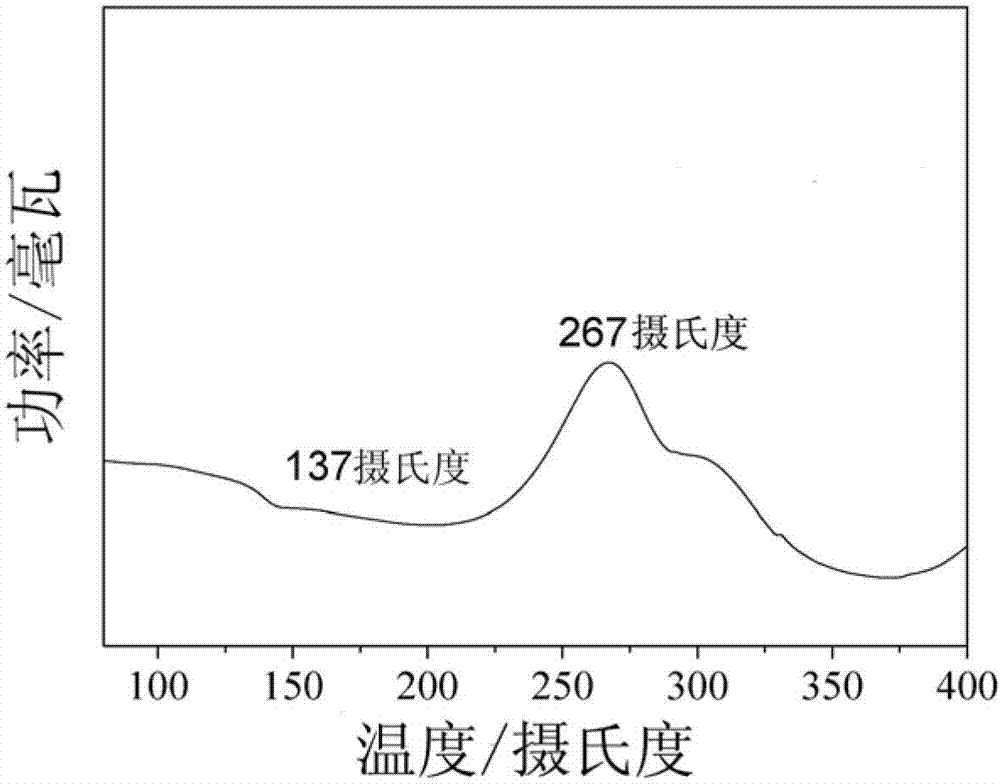
[0056] figure 1 The DSC curve of the polyaryl ether sulfon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com