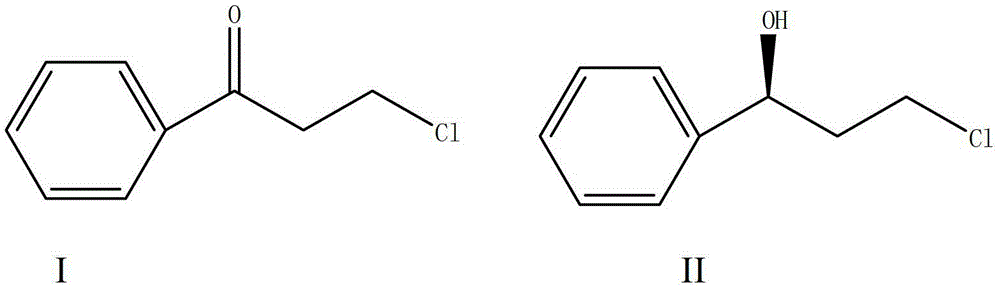
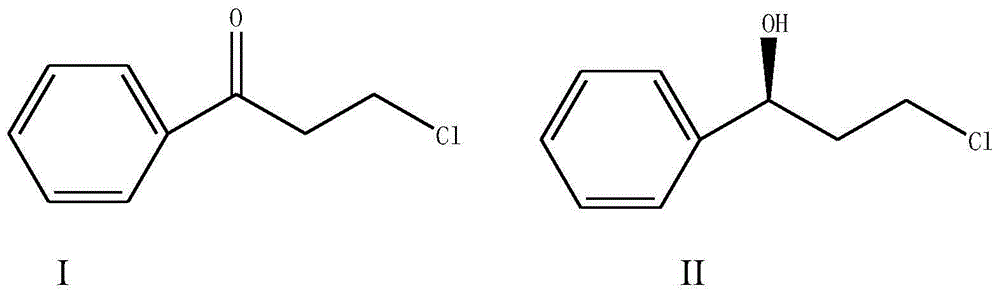
Magnetic porous supported metallic chiral catalyst and application thereof
A chiral catalyst, supported technology, used in physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] a), 1.0mmol FeCl 3 ·6H 2 O, 1.5mmol C 6 h 5 o 7 Na 3 2H 2 O, 2.0 mmol of urea and 1.0 g of PAM were added to 10 mL of distilled water with stirring, and after it was completely dissolved in water, the solution was transferred to a hydrothermal reaction kettle, sealed, heated to 150°C, and reacted for 8 hours. The obtained black precipitate was separated by centrifugation, washed three times with distilled water and absolute ethanol respectively, and dried in a vacuum drying oven for later use to obtain ferric oxide nanospheres.
[0053]b) Disperse the self-made iron ferric oxide nanospheres (0.6 g) in 20 mL of HCl (0.1 M) aqueous solution, and ultrasonically disperse in an ice bath for 10 min. Then ferric oxide nano-microspheres were magnetically separated, washed with distilled water for 3 times, and then mixed with a mixed solvent of 80 mL of ethanol, 16 mL of distilled water and 0.16 mL of ammonia water. The resulting mixed solution was ultrasonicated in an ic...
Embodiment 2
[0058] a), 1.5mmol FeCl 3 ·6H 2 O, 2.0 mmol C 6 h 5 o 7 Na 3 2H 2 O, 3.0 mmol of urea and 1.25 g of PAM were added to 15 mL of distilled water with stirring, and after it was completely dissolved in water, the solution was transferred to a hydrothermal reaction kettle, sealed, heated to 170°C, and reacted for 12 hours. The obtained black precipitate was separated by centrifugation, washed several times with distilled water and absolute ethanol respectively, and dried in a vacuum drying oven for later use to obtain ferric oxide nanospheres.
[0059] b) Disperse the self-made iron ferric oxide nanospheres (0.7 g) in 25 mL of HCl (0.1 M) aqueous solution, and ultrasonically disperse in an ice bath for 15 min. Then ferric oxide nano-microspheres were magnetically separated, washed 3 times with distilled water, and then mixed with 100 mL of ethanol, 25 mL of distilled water and 0.25 mL of ammonia water. The resulting mixed solution was ultrasonicated for 15 min in an ice bat...
Embodiment 3
[0065] a), 2.0mmol FeCl 3 ·6H 2 O, 2.5mmol C 6 h 5 o 7 Na 3 2H 2 O, 4.0mmol urea and 1.5g PAM were added to 20mL of distilled water under stirring conditions, and after it was completely dissolved in water, the solution was transferred to a hydrothermal reaction kettle, sealed, heated to 180°C, and reacted for 16h. The obtained black precipitate was separated by centrifugation, washed several times with distilled water and absolute ethanol respectively, and dried in a vacuum drying oven for later use to obtain ferric oxide nanospheres.
[0066] b) Disperse the self-made iron ferric oxide nanospheres (0.8 g) in 30 mL of HCl (0.1 M) aqueous solution, and ultrasonically disperse in an ice bath for 20 min. Then ferric oxide nano-microspheres were magnetically separated, washed three times with distilled water, and then mixed with 120 mL of ethanol, 36 mL of distilled water and 0.36 mL of ammonia water. The resulting mixed solution was ultrasonicated for 20 min in an ice bat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com