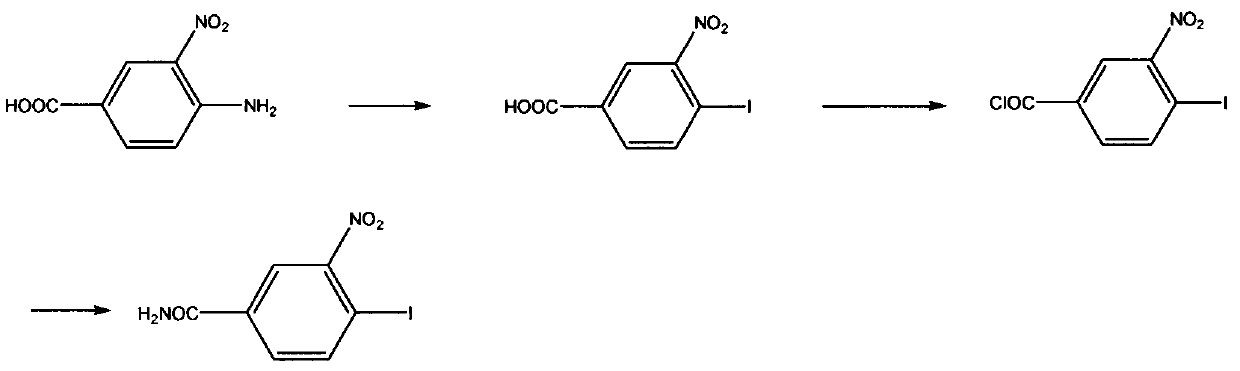
Preparation method and key intermediate of antineoplastic reagent 4-iodo-3-nitrobenzamide
A technology of nitrobenzamide and intermediates, applied in the field of preparation of 4-iodo-3-nitrobenzamide, can solve the problems of low yield, poor reaction selectivity, low purity, etc., and achieve increased yield and The effect of purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
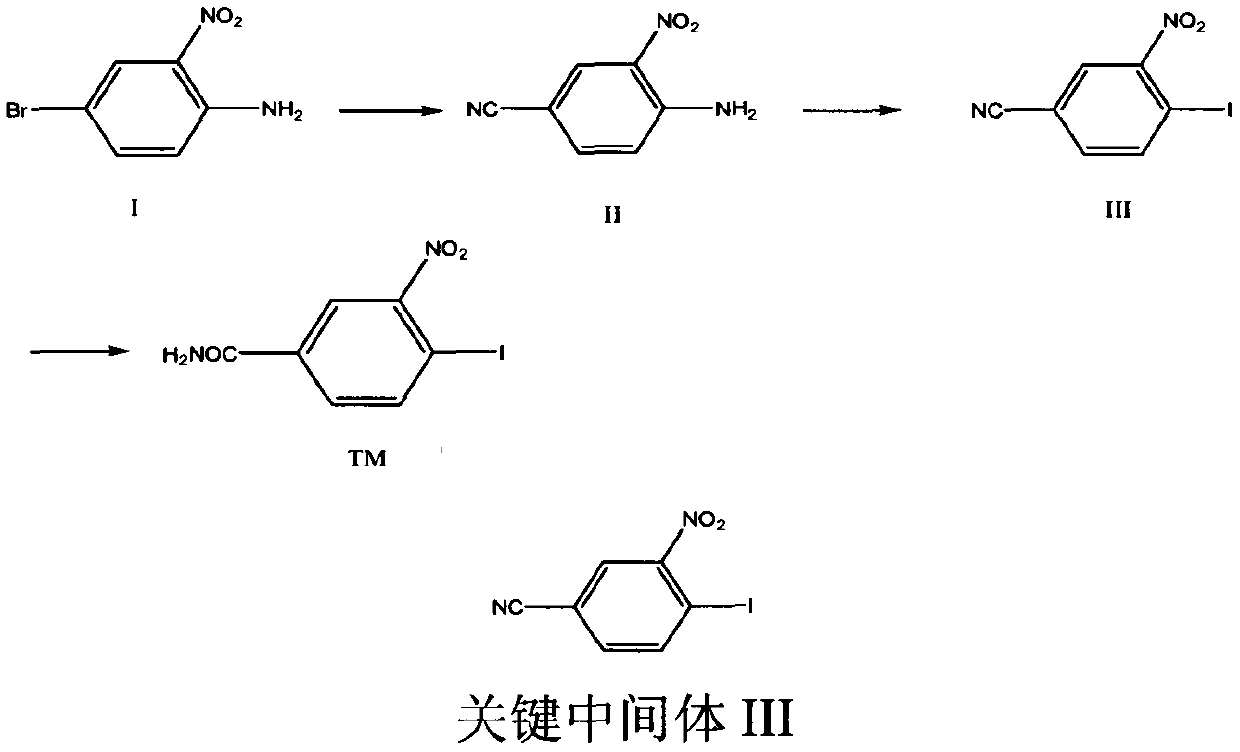
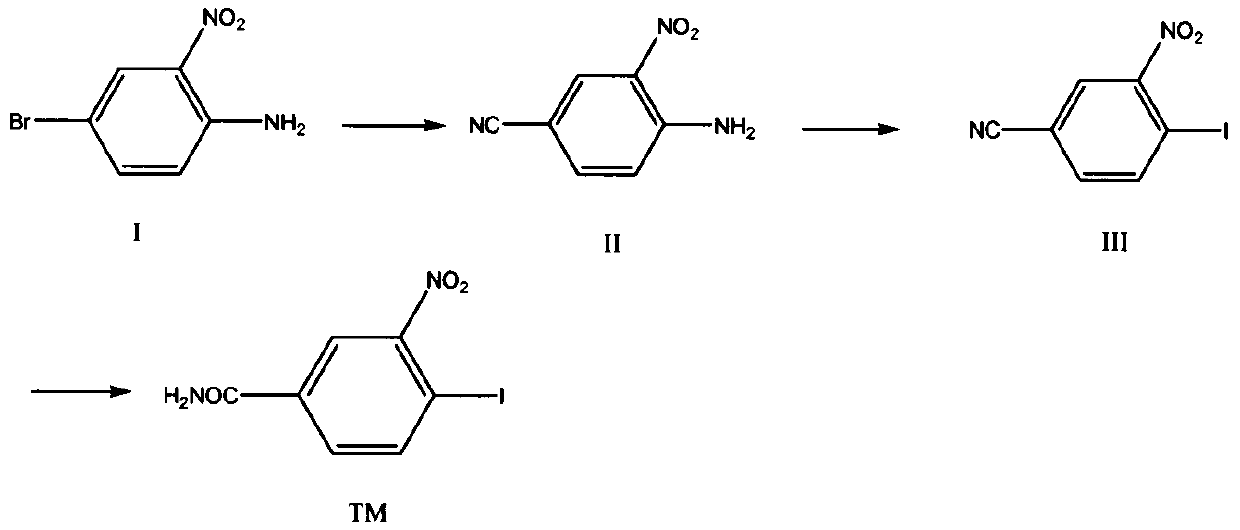
[0029] Example 1: Preparation of 4-cyano-2-nitroaniline (compound II).
[0030]
[0031] In a 500ml three-necked flask, add 21.6g (0.1mol) of compound I, 10g (0.12mol) of cuprous cyanide, and 200ml of DMF, stir, heat up to 60°C and react for 5h, TLC detection shows that the reaction is complete, and cool to room temperature , distilled, and the solvent was removed to obtain a residue, which was extracted with ethyl acetate (20ml×3), distilled under reduced pressure, and recrystallized from ethanol to obtain 14.5g of a reddish solid, yield: 70.2%.
[0032] MS (+1): 164.
[0033] 1H NMR: d (ppm, CDCl3), 8.19 (s, 1H), 7.64-7.65 (d, 1H), 6.92-6.93 (d, 1H), 4.237-4.24 (br, 2H).
Embodiment 2
[0034] Embodiment 2: Preparation of 4-iodo-3-nitrobenzonitrile (compound III)
[0035]
[0036] Add 32.6g of Intermediate II (0.2mol), 540mL of distilled water, and 50mL of concentrated hydrochloric acid into a 2L three-neck flask, stir mechanically, cool down to 0-5°C in an ice bath, and the reaction solution is a yellow slurry. Dissolve 20.7g of sodium nitrite (0.3mol) in 230mL of water, and slowly add it dropwise to the above reaction system, some solids will dissolve, control the temperature in the system at 0-5°C, continue stirring to complete the addition, and control the temperature to react for 3h. Filtrate with suction to obtain a yellow solid and filtrate, and transfer the filtrate to a 3L three-neck flask. Dissolve 66g of potassium iodide (0.4mol) in 148mL of water, slowly add it dropwise into the reaction system, a large amount of air bubbles are generated, after the addition, continue to stir for 2h, and filter with suction to obtain an orange-red filter cake, ...
Embodiment 3
[0039] Embodiment 3: the preparation of 4-iodo-3-nitrobenzamide (compound TM)
[0040]
[0041] In a 250ml three-neck flask, add 27.5g (0.1mmol) of compound III and 200ml of concentrated hydrochloric acid, stir, heat up to 80°C for 5 hours, TLC spotting test shows that the reaction is complete, cool to room temperature, precipitate solid, suction filter, vacuum dry , to obtain 26.3 g of white solid, yield: 90.1%.
[0042] MS (+1): 293.
[0043] HNMR: d (ppm, CDCl3), 8.65 (s, 1H), 8.11-8.12 (d, 1H), 8.08-8.09 (d, 1H), 6.12-6.13 (s, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com