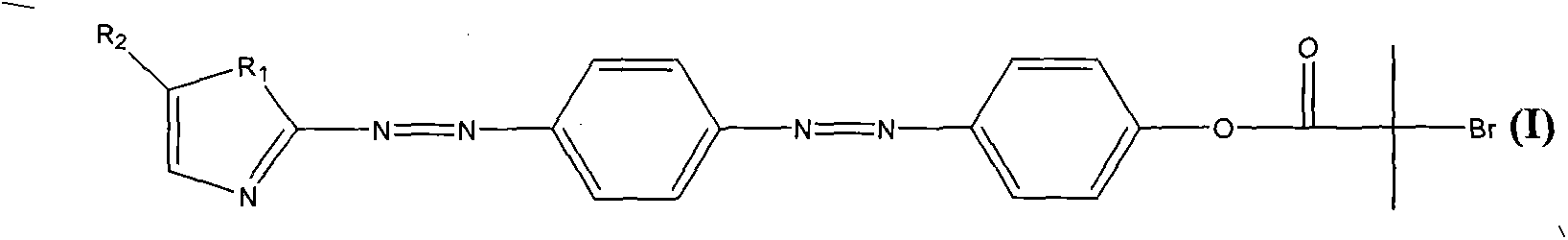
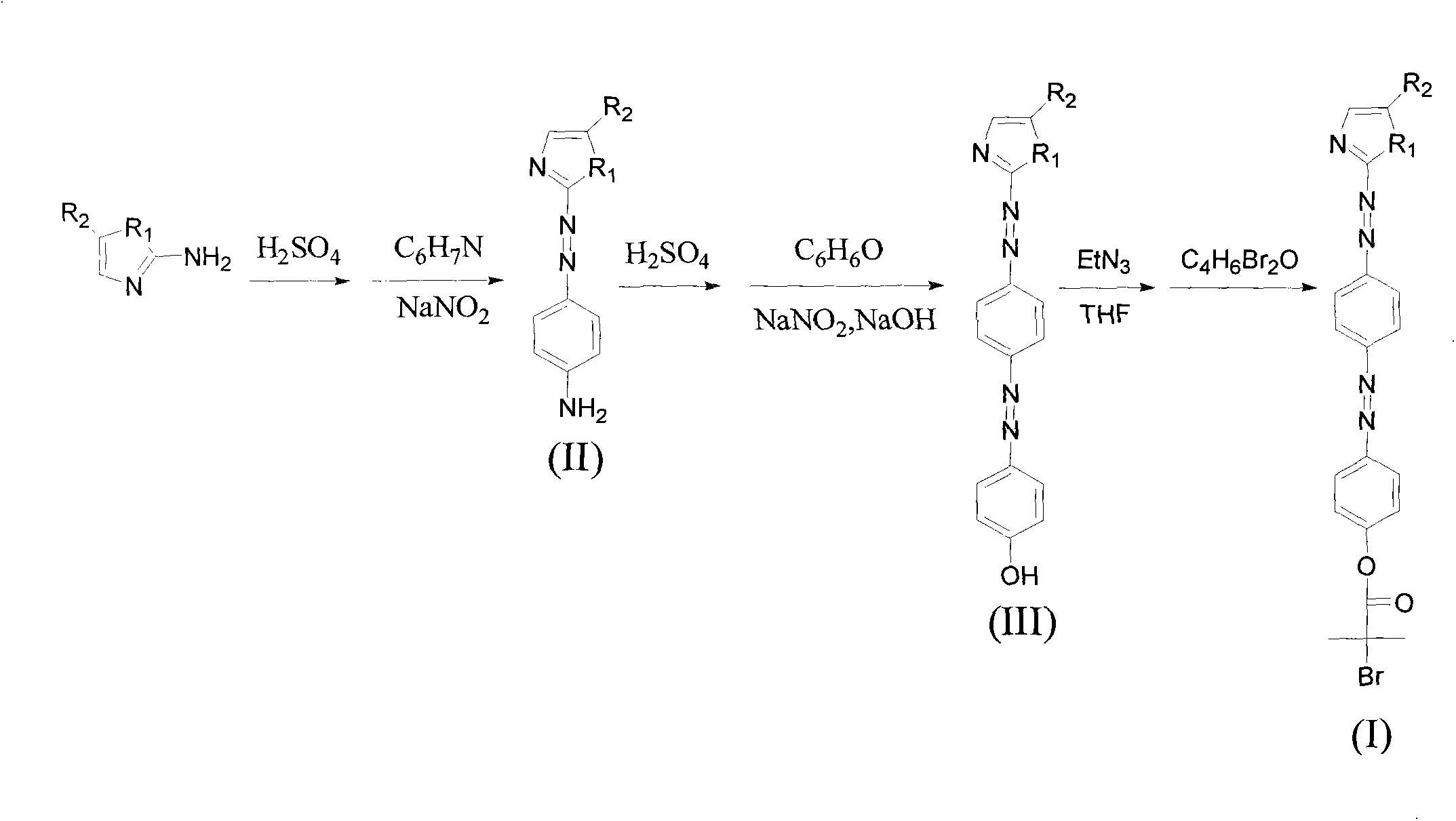
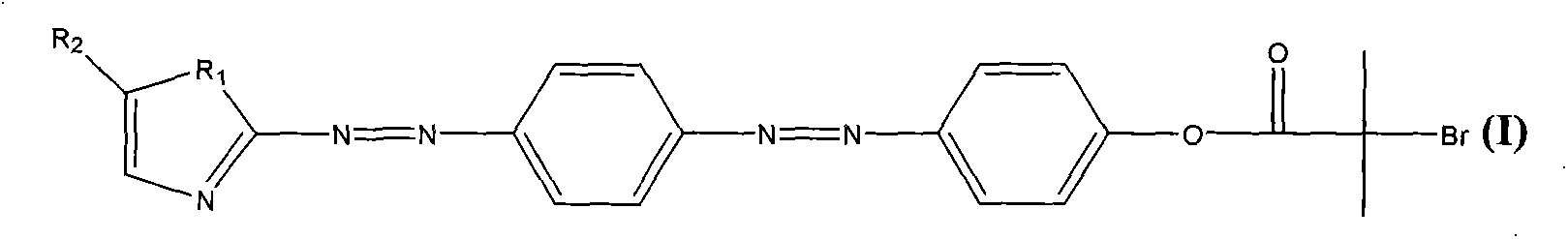
Compound containing pentazole ring bisazo structure, as well as preparation method and application thereof
A technology of azole ring bisazo and azole ring azo is applied in the field of compounds containing five-membered azole bicyclic azo structures, and can solve the problem that the coverage of chromophore bopp is not wide enough, the monomer conversion rate is not high enough, and the polymerization rate is not enough. It is not fast enough to achieve the effects of increasing solubility and catalytic performance, broadening the range of chromophores, and narrowing the molecular weight distribution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Take 0.5-3g of 2-aminoimidazole to make a sulfate solution, and cool it in an ice bath. Take 0.5-2g of aniline and 0.5-2g of sodium nitrite, add hydrochloric acid aqueous solution to form a solution, and cool it to below 2°C with an ice bath. The aniline solution was slowly added dropwise to the sulfate solution with stirring while keeping the temperature below 2 °C. After the dropwise addition, continue to stir at below 5°C for 5 hours, adjust to neutrality with ammonia water, filter the reaction solution with suction and wash with a large amount of deionized water to obtain an intermediate containing an azo structure of an imidazole ring.
[0031] Take 0.5-3g of the intermediate containing the imidazole ring azo structure to make a sulfate solution, and cool it in an ice bath. Take 0.5-2g of phenol and 0.5-2g of sodium nitrite, add sodium hydroxide aqueous solution to form a solution, and cool it to below 2°C with an ice bath. The phenol solution was slowly added dr...
Embodiment 2
[0035] Take 0.5-3g of 2-amino-5-nitro-imidazole to make a sulfate solution, and cool it in an ice bath. Take 0.5-2g of aniline and 0.5-2g of sodium nitrite, add hydrochloric acid aqueous solution to form a solution, and cool it to below 2°C with an ice bath. The aniline solution was slowly added dropwise to the sulfate solution with stirring while keeping the temperature below 2 °C. After the dropwise addition, continue to stir at below 5°C for 5 hours, adjust to neutrality with ammonia water, filter the reaction solution with suction and wash with a large amount of deionized water to obtain an intermediate containing 5-nitroimidazole ring azo structure.
[0036] Take 0.5-3g of the intermediate containing 5-nitroimidazole ring azo structure to prepare a sulfate solution, and cool it in an ice bath. Take 0.5-2g of phenol and 0.5-2g of sodium nitrite, add sodium hydroxide aqueous solution to form a solution, and cool it to below 2°C with an ice bath. The phenol solution was sl...
Embodiment 3
[0040] Take 0.5-3g of 2-amino-5-methoxy-imidazole to make a sulfate solution, and cool it in an ice bath. Take 0.5-2g of aniline and 0.5-2g of sodium nitrite, add hydrochloric acid aqueous solution to form a solution, and cool it to below 2°C with an ice bath. The aniline solution was slowly added dropwise to the sulfate solution with stirring while keeping the temperature below 2 °C. After the dropwise addition, continue to stir at below 5°C for 5 hours, adjust to neutrality with ammonia water, filter the reaction solution with suction and wash with a large amount of deionized water to obtain an intermediate containing 5-methoxyimidazole ring azo structure.
[0041] Take 0.5-3g of the intermediate containing 5-methoxyimidazole ring azo structure to make a sulfate solution, and cool it in an ice bath. Take 0.5-2g of phenol and 0.5-2g of sodium nitrite, add sodium hydroxide aqueous solution to form a solution, and cool it to below 2°C with an ice bath. The phenol solution was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com