Preparation method of 12-tetradecadienyl acetate of corn borer sex pheromone
A technology of tetradecenol acetate and corn borer, which is applied in the preparation of carboxylic acid esters, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of harsh reaction conditions and low reaction selectivity, and achieves Fewer side reactions, simple and reasonable steps, and the only effect of the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
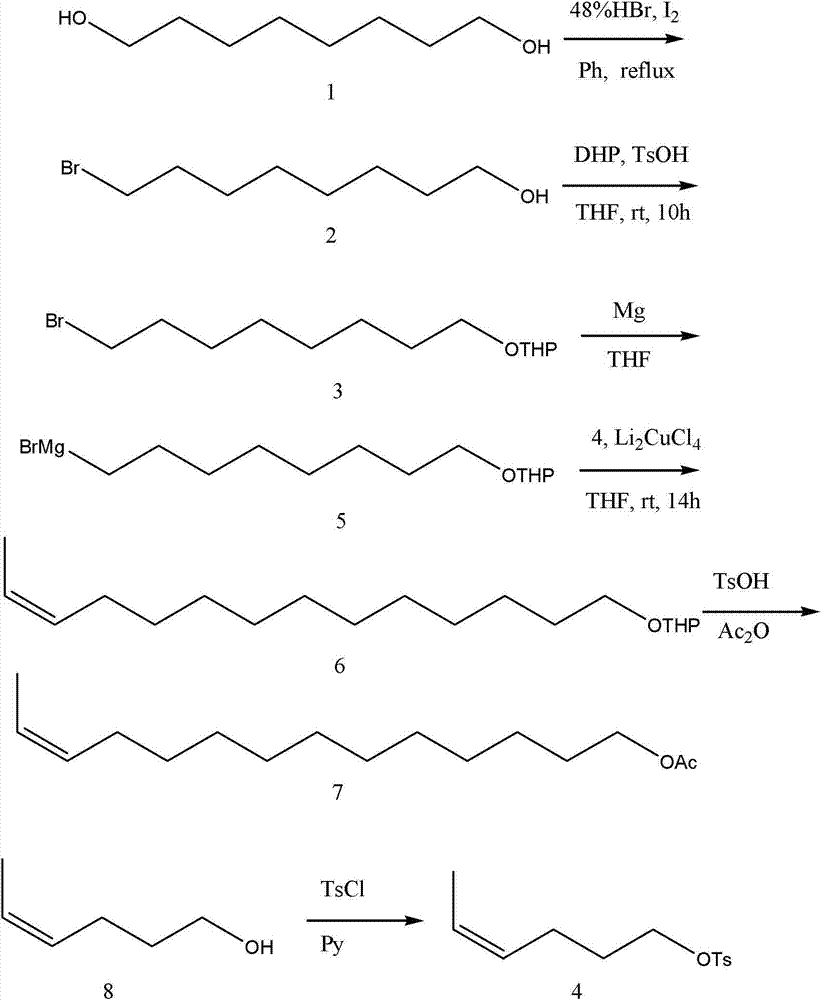
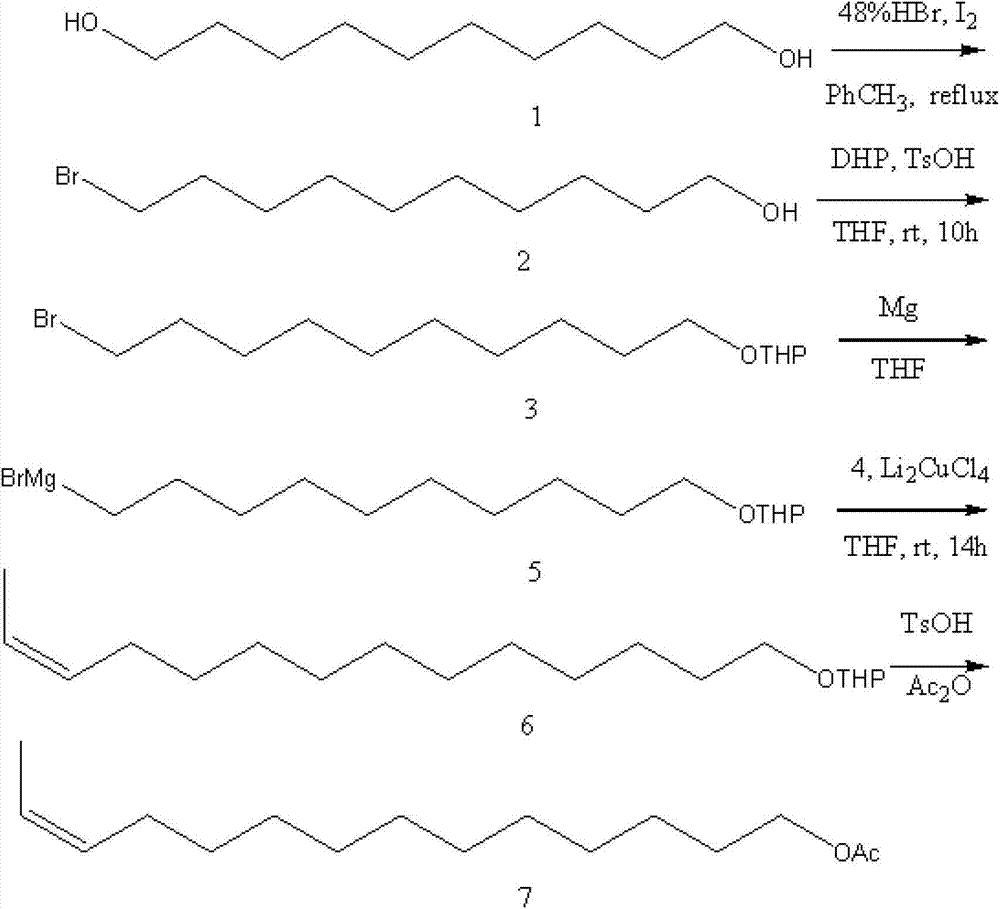
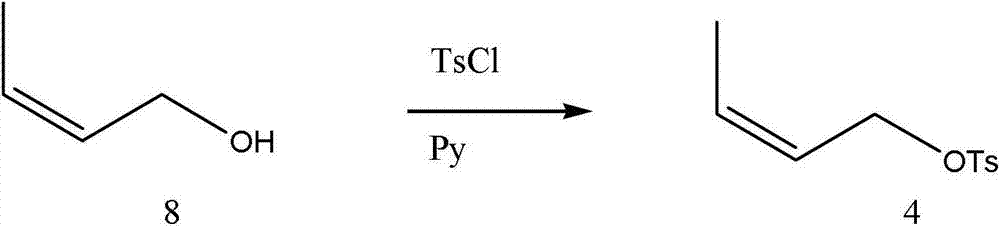
[0033] Specific embodiment 1: Z-12-tetradecenyl acetate in the corn borer sex pheromone is based on cis-4-hexen-1-ol and 1,8-octanediol as starting materials The raw material 1,8-octanediol is unilaterally brominated to obtain 8-bromo-1-octanol, and then protected by 3,4-dihydropyran to obtain 8-bromo-1-octanol tetrahydropyran, Then react with magnesium to get 8-magnesium bromide-1-octanol tetrahydropyran; the raw material cis-4-hexene-1-alcohol reacts with p-toluenesulfonic acid chloride to get cis-4-hexene-1-p Grignard coupling reaction of benzenesulfonate, cis-4-hexene-1-p-toluenesulfonate and 8-magnesium bromide-1-octanol tetrahydropyran to obtain Z-12-tetradecene tetrahydro Pyran, then through deprotection, esterification to obtain the Z-12-tetradecenol acetate in the sex pheromone of Ostrinia sativa; the specific operation is as follows:
[0034] The first step: the preparation method of 8-bromo-1-octanol is: add 0.1mol (14.6g) 1,8-octanediol, 300mL of benzene and 0.1m...
specific Embodiment approach 2
[0040] Specific embodiment two: E-12-tetradecenyl acetate in the corn borer sex pheromone is based on crotyl alcohol and 1,10-decanediol as starting materials, wherein the raw material 1,10-decenyl Diol is unilaterally brominated to obtain 10-bromo-1-decyl alcohol, then protected by 2,3-dihydropyran to obtain 10-bromo-1-octanol tetrahydropyran, and then reacted with magnesium to obtain 10 -magnesium bromide-1-octanol tetrahydropyran; raw material crotyl alcohol reacts with p-toluenesulfonic acid chloride to obtain crotyl alcohol p-benzenesulfonate, crotyl alcohol p-benzenesulfonate and 10-magnesium bromide-1 -Octanol tetrahydropyran and E-12-tetradecene tetrahydropyran after Grignard coupling reaction, and then deprotection and esterification of the E-12-tetrahydropyran in the sex pheromone of the Asian corn borer Carbenol acetate; Specifically, carry out according to the following steps:
[0041] Step 1, the preparation method of described 10-bromo-1-decyl alcohol is as foll...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com