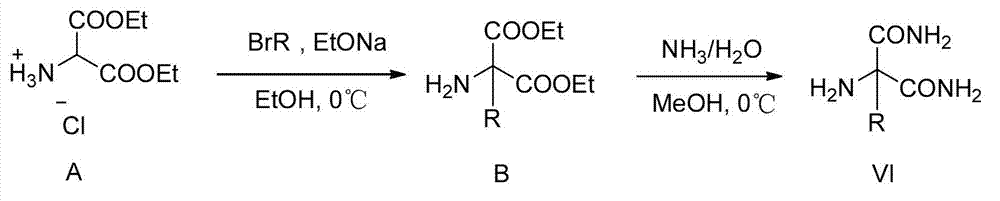
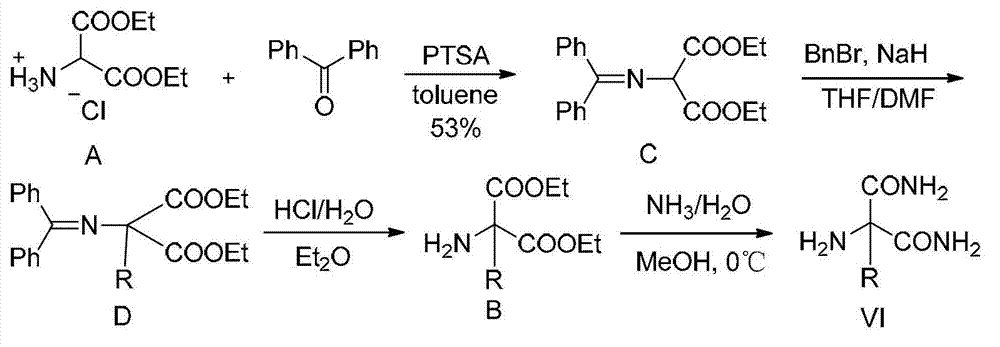
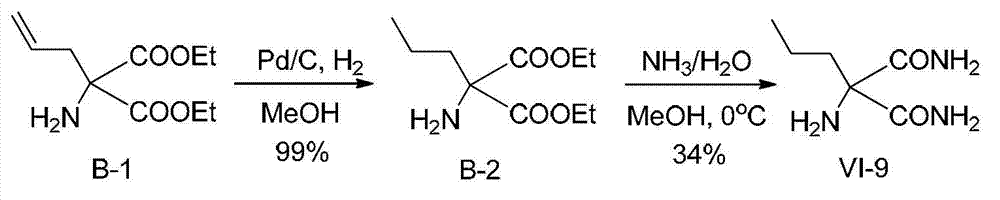
Functionalized unnatural amino acids with quaternary carbon centers and biocatalytic desymmetrization preparation method thereof
A compound and catalytic hydrolysis technology, applied in biochemical equipment and methods, microorganism-based methods, microorganisms, etc., can solve problems such as poor selectivity and harsh chemical transformation requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077]
[0078] Specific implementation method:
[0079] Get 2 grams of wet weight of Rhodococcus erythropolis AJ270 cells (Institute of Chemistry, Chinese Academy of Sciences), thaw at 30°C for 30 minutes, and use a buffer solution of dipotassium hydrogen phosphate and potassium dihydrogen phosphate (0.1M, pH7.0, 50ml) Wash the bacterium into an Erlenmeyer flat-bottomed flask with a threaded opening, disperse and shake well, and then put it in a shaker for activation at 30°C for 30 minutes, then add 2mmol (314mg) of the compound shown in formula VI-1 at one time, put The catalytic hydrolysis reaction was carried out in a shaker at 30° C. and 200 rpm. Whole reaction TLC monitors, stops reaction after reacting for 40 minutes, gained reaction solution removes thalline through one deck diatomite suction filtration, washes filter residue three times with 20 milliliters of water successively, obtains the compound shown in formula 1 provided by the invention (R 1 for -CH 2 CH=C...
Embodiment 2
[0083]
[0084] Specific implementation method:
[0085] Compound 1 (100mg) was added to DMF (5ml), K 2 CO 3 (1.5mmol), benzyl bromide (2mmol) and stirred at room temperature (25°C) overnight (12 hours) to complete the reaction. followed by H 2 O (25ml), extracted with ethyl acetate (3×25ml), anhydrous MgSO 4 Drying, remove solvent with rotary evaporator, dry loading, flash column chromatography obtains the compound described in formula I-2 provided by the invention (R 1 for -CH 2 CH=CH 2 , R 2 for-NH 2 , R 3 -Bn) 196 mg, yield: 91%, ee97.0%.
[0086] Compound 2 solid, mp145-146°C; [α] 25 D : +17.9° (c1.90, CHCl 3 ); ee=97.0% (chiral HPLC analysis); IR (KBr) v3437cm -1 , 1742cm -1 , 1689cm -1 , 1202cm -1 ; 1 H NMR (300MHz, CDCl 3 )7.32-7.19 (m, 10H), 7.08 (s, 1H), 5.80-5.66 (m, 2H), 5.29-5.13 (m, 4H), 3.61 (dd, J=26.7, 12.0Hz, 2H), 2.97 -2.84(m, 2H)2.31(s, 1H); 13 C NMR (75MHz, CDCl 3 ) 172.2, 170.7, 135.4, 131.5, 128.63, 128.58, 128.51, 128.47, 128.2, ...
Embodiment 3
[0089]
[0090] 1) Take two grams of wet weight of Rhodococcus erythropolis AJ270 cells (Institute of Chemistry, Chinese Academy of Sciences), thaw at 30°C for 30 minutes, and use a buffer solution of dipotassium hydrogen phosphate and potassium dihydrogen phosphate (0.1M, pH7.0, 50ml) and wash the bacterium into a 150ml Erlenmeyer flat-bottomed flask with a screw top, disperse and shake well, put it into a shaker and activate it at 30°C for 30 minutes, then add 2mmol (342mg) of the formula VI-2 at one time The compound was placed in a shaker at 30° C. and 200 rpm to carry out catalytic hydrolysis reaction. Whole reaction TLC monitors, stops reaction after reacting 50min, and gained reaction solution removes thalline through one layer of diatomaceous earth suction filtration, washes filter residue three times with 20 milliliters of water successively, obtains the compound shown in formula 3 provided by the invention (R 1 for -CH 2 CH=CHCH 3 , R 2 for-NH 2 ) 320 mg, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com