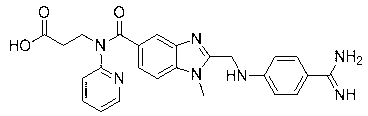
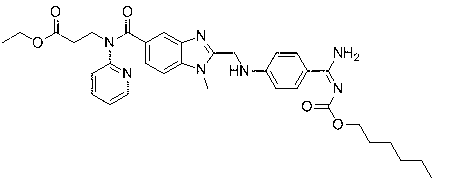
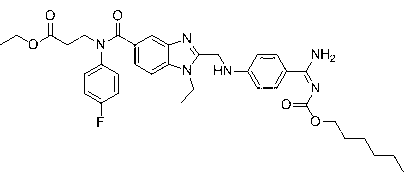
Fluorine group-containing-modified dabigatran etexilate analogue and synthetic method thereof
A dabigatran etexilate and synthetic method technology, applied in the direction of organic chemistry, etc., can solve the problems of high price, increased preparation cost of dabigatran etexilate analogues, increased risk of preparation process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] A fluorine-containing modified dabigatran etexilate analogue, namely 3-[[[2-[[[4-[[[(hexyloxy)carbonyl] amino] iminomethyl] phenyl] amino] methyl Base]-1-ethyl-1H-benzimidazol-5-yl]carbonyl](4-fluorophenyl)amino]propionic acid ethyl ester, that is, 4-trifluoroaniline as raw material, synthesized through 9 steps , the final product contains fluorine-modified dabigatran etexilate analogs, i.e. 3-[[[2-[[[4-[[[(hexyloxy)carbonyl]amino]iminomethyl]phenyl]amino] Methyl]-1-ethyl-1H-benzimidazol-5-yl]carbonyl](4-fluorophenyl)amino]ethyl propionate, the synthetic reaction process schematic diagram is as follows Figure 4 As shown, each step of synthesis reaction is as follows:
[0058] (1), Synthesis of ethyl 3-(4-fluorophenylamino)propionate
[0059] Add 4-fluoroaniline (5.57g, 50.0mmol), ethyl 3-bromopropionate (16.45g, 90.0mmol) and triethylamine (21.0mL) into a round bottom flask, toluene as solvent, and react at 100°C for 12h Obtain reaction solution 1;
[0060] The 4-f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com