Veterinary procaine penicillin-dihydrostreptomycin sulfate suspension injection and preparation method thereof
A technology of dihydrostreptomycin sulfate and procaine penicillin, which is applied to medical preparations containing active ingredients, liquid delivery, and pharmaceutical formulations, can solve the problems of technical disclosure of the combination of two drugs, and achieve Effects of improving convenience, expanding antibacterial spectrum, and facilitating clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
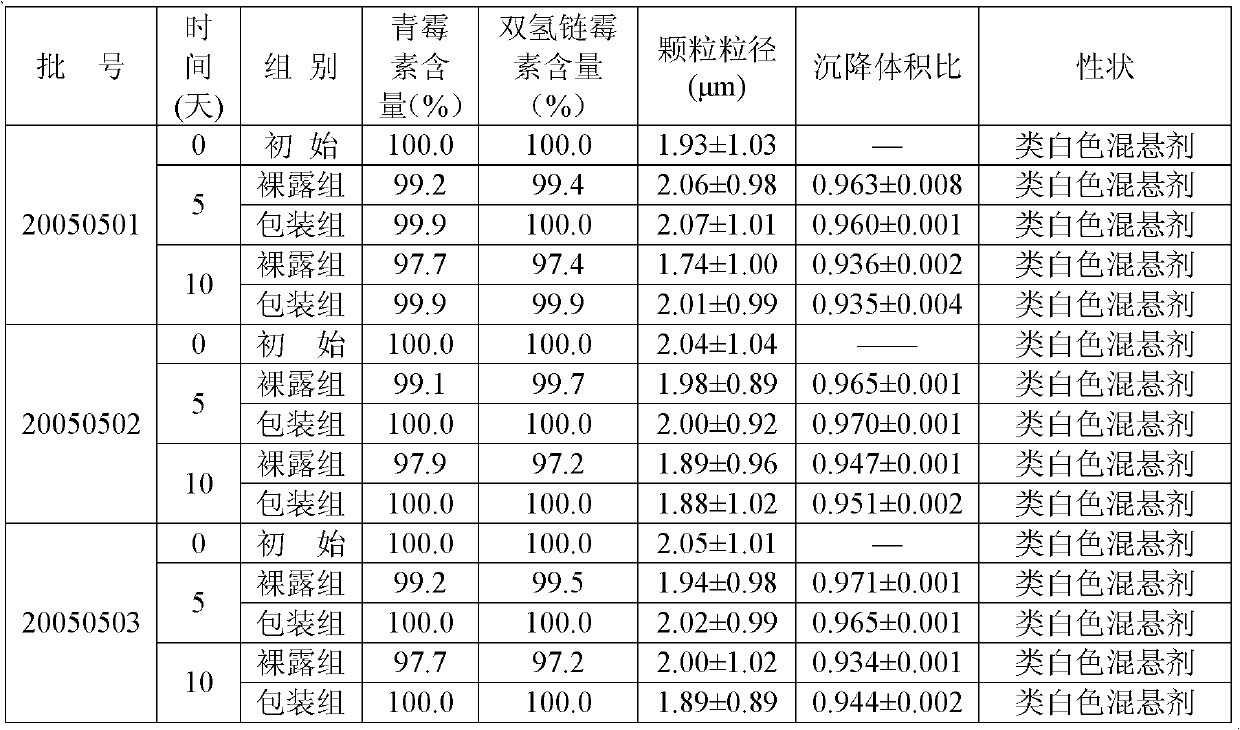
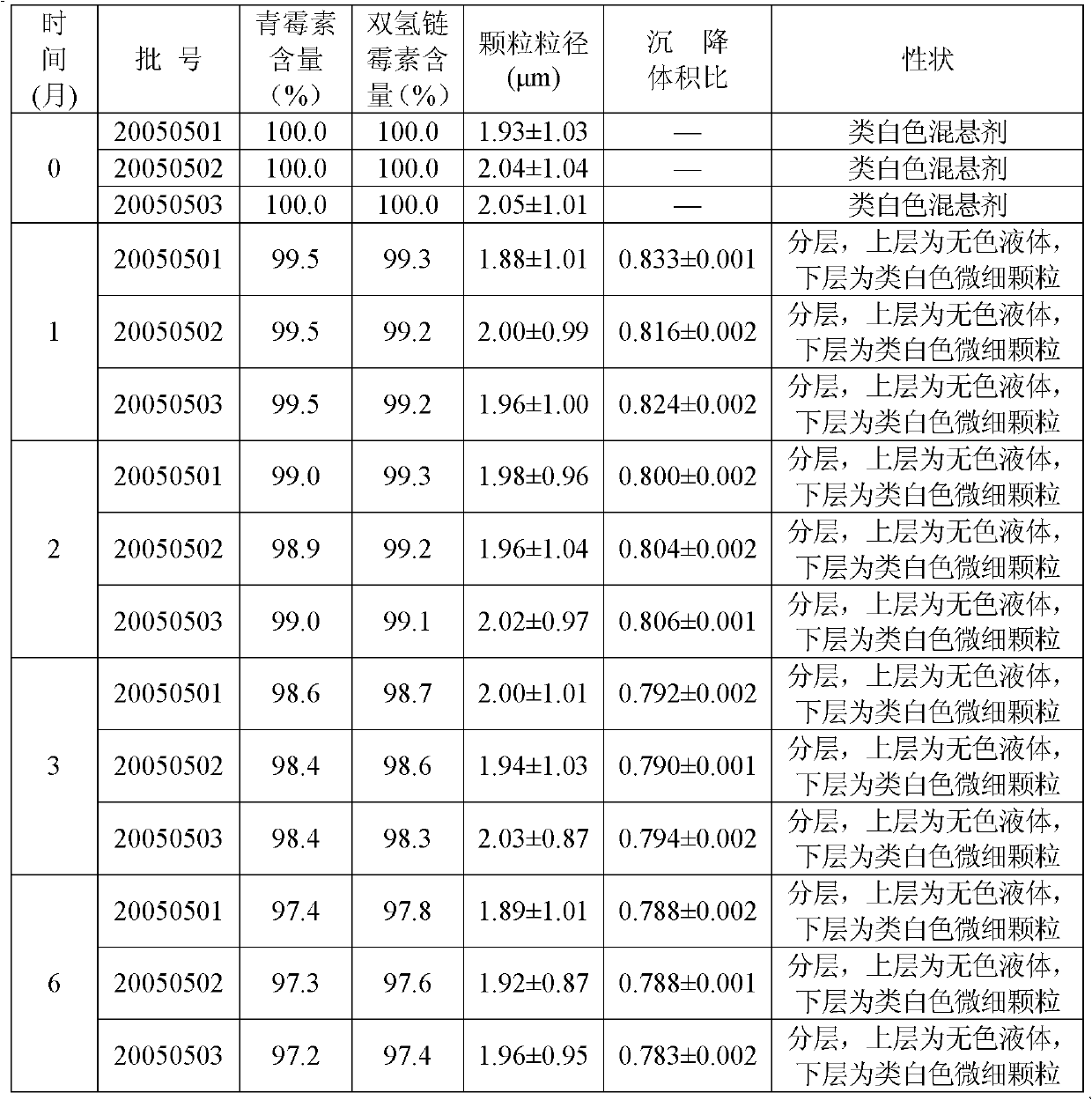
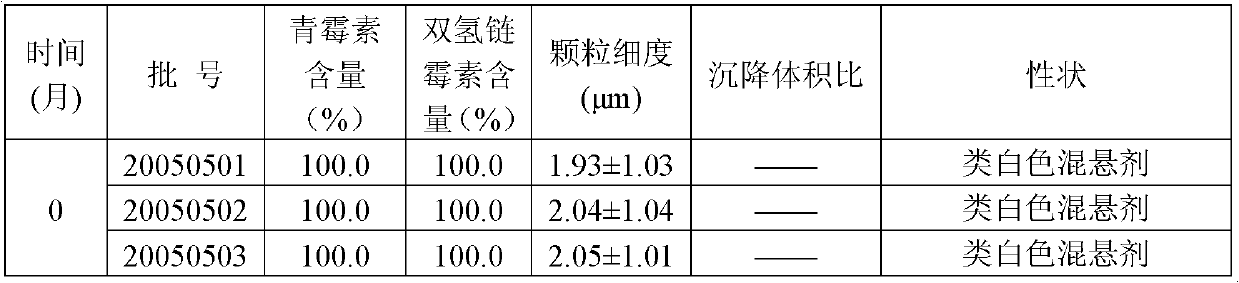
[0027] Procaine penicillin 5g, dihydrostreptomycin sulfate 5g, appropriate amount of citrate buffer, sodium methylparaben 0.02g, polyvinylpyrrolidone 0.5g, sodium carboxymethylcellulose 0.2g, Promethacin hydrochloride Caine 0.5g, sodium dithionite 0.02g, and water for injection were added to 100ml.
[0028] Preparation method: Take 50ml of water for injection, add suspending agent, soak for 8 hours, add polyvinylpyrrolidone to disperse evenly, add procaine penicillin, stir and mix evenly, add preservative dissolved in 20ml of water for injection, procaine hydrochloride Because of sodium dithionite, add buffer solution, adjust the pH value to about 7.0, constant volume, under the set pressure of the equipment 25Mpa, pass through the homogenizer twice, mix evenly, and pack it. The pressure and pH value are only the setting indexes of the operation, and the requirements are not strict, and fluctuations within the scope defined by the present invention are all allowed.
Embodiment 2
[0030] Procaine penicillin 10g, dihydrostreptomycin sulfate 10g, appropriate amount of acetate buffer, methylparaben 0.5g, polyvinylpyrrolidone 2.5g, carboxymethylcellulose sodium 0.3g, procaine hydrochloride 1.0g, 0.3g of sodium dithionite, and water for injection to 100ml.
[0031] The preparation method is the same as in Example 1.
Embodiment 3
[0033] Procaine penicillin 20g, dihydrostreptomycin sulfate 20g, appropriate amount of phosphate buffer, propylparaben 1.05g, polyvinylpyrrolidone 10g, carboxymethylcellulose 0.4g, procaine hydrochloride 1.5g, Sodium dithionite 0.35g, add water for injection to 100ml.
[0034] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com