Lyophilized vaccine for swine encephalitis B and preparation method thereof
A technology for porcine Japanese encephalitis and vaccines, which is applied in the direction of medical formulas, medical preparations with non-effective ingredients, and non-effective ingredients of high-molecular compounds, and can solve the problem of poor storage effect, low storage temperature, and Energy waste and other issues, to achieve the effects of simple formula ingredients and preparation methods, good heat resistance, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
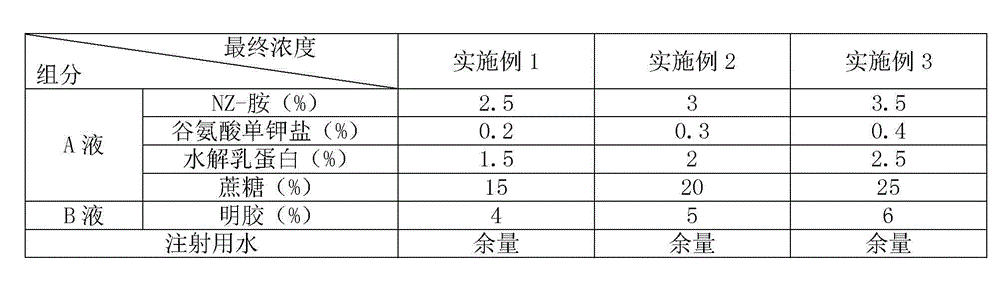
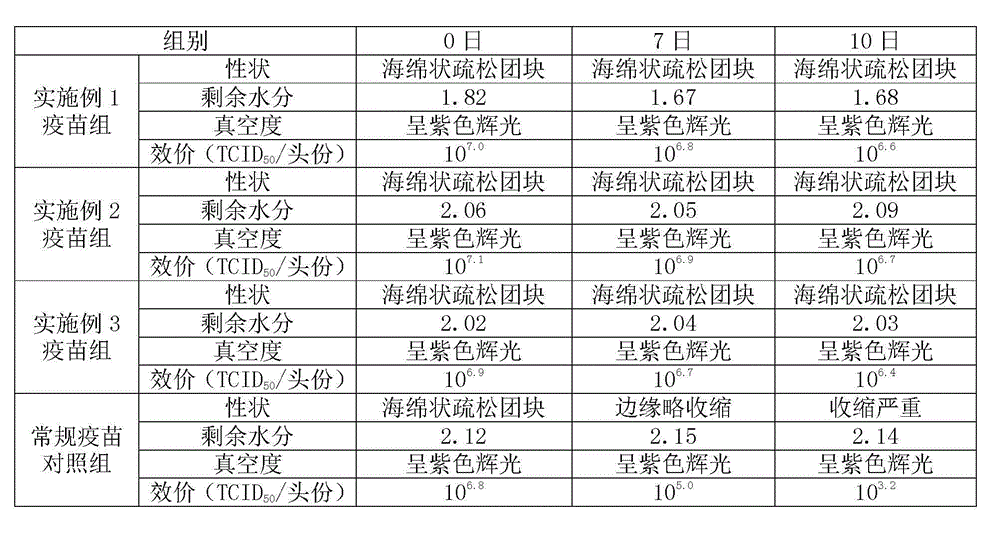
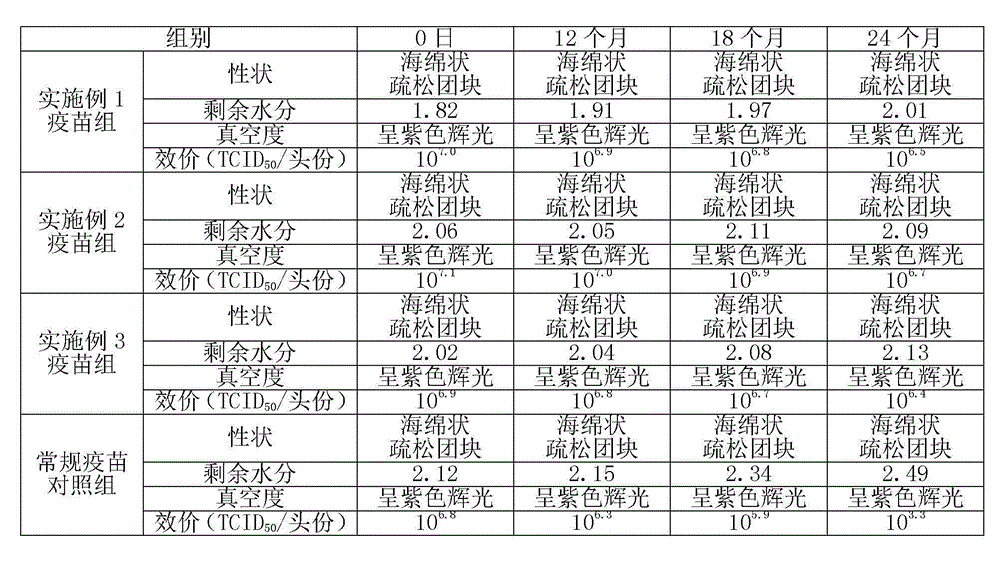
[0012] In the field of vaccine lyoprotectant preparation, the selection and ratio of the protective agent components have an important impact on the preservation effect of the vaccine. In order to enable the swine fever vaccine of the present invention to be preserved for a long time, the applicant has carried out long-term research on the components of the lyoprotectant of the present invention, and optimized the proportioning concentrations of each other, so that each component has the greatest complex matching effect.
[0013] For the used components of the lyoprotectant of the present invention, its properties are as follows:
[0014] 1. NZ-Amine AS, also known as the enzymatic hydrolyzate of casein;
[0015] 2. Glutamic acid monopotassium salt (L-glutamic acid potassium salt): It is a surfactant that can reduce the interfacial tension. Dehydrated and deformed, it can act as a wetting agent for active components during rehydration.
[0016] 3. Hydrolyzed milk protein, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com