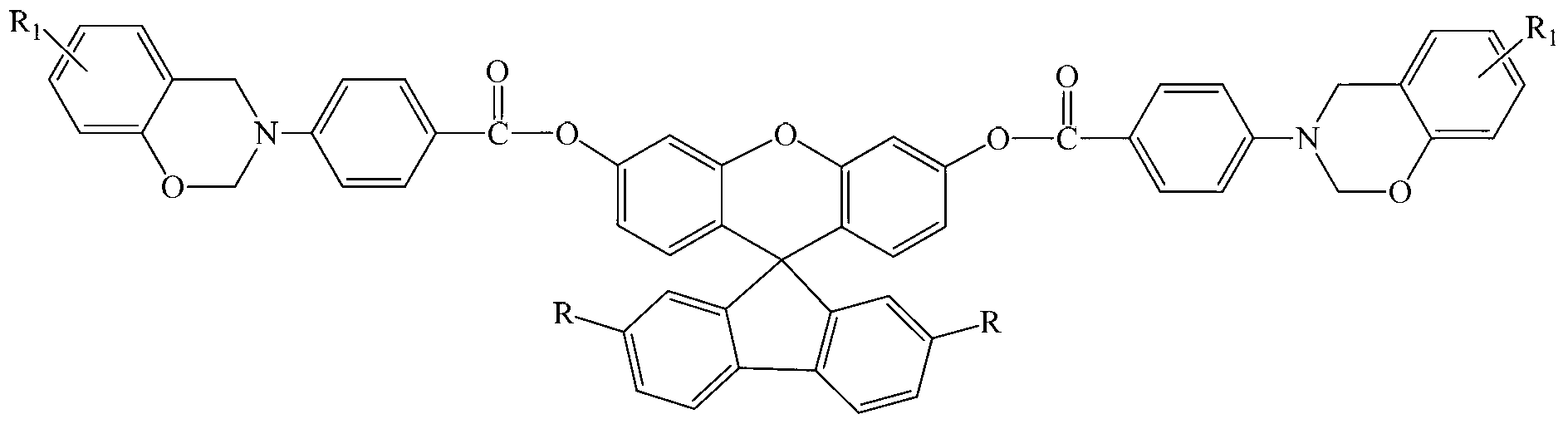
Spirofluorene xanthene benzoxazine containing benzoyloxy group and preparation method thereof
A technology of benzoyloxyspirofluorenexanthene benzene and nitrobenzoyloxyspirofluorenexanthene, which is applied in the field of polymer materials and can solve the problems of increased polymer brittleness, high molecular chain rigidity, and toughness. Improve processing performance, improve flexibility, and lower melting point
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Step 1: Add 5.0 g (13.7 mmol) of 3',6'-dihydroxy-spiro(fluorene-9,9'-xanthene) to a 250 mL three-necked flask equipped with a stirrer, dropping funnel and thermometer ), 50mL of anhydrous chloroform and 50mL of triethylamine, and then add dropwise 50mL of 4-nitrobenzoyl chloride (5.25g, 28.3mmol) chloroform solution at 2-4°C under stirring conditions. Continue to react under the conditions for 3 hours, raise the temperature, and react at reflux temperature for 16 hours. After the reaction, the solvent is evaporated under reduced pressure, and then washed with water, washed with ethanol, filtered, and dried in vacuum to obtain 7.04g of light yellow 3',6'- Bis(4-nitrobenzoyloxy)-spiro(fluorene-9,9'-xanthene) powder, yield 77.6%.
[0021] Step 2: Into a 150mL three-neck flask equipped with a stirrer, dropping funnel and thermometer, add 3',6'-bis(4-nitrobenzoyloxy)-spiro(fluorene-9 , 9'-oxanthene) 4.5g (6.8mmol), 0.4g palladium / carbon and 80mL of absolute ethanol, dropwis...
Embodiment 2
[0026] Except that the raw material phenol in the third step is replaced by m-cresol, and the dosage is 2.16g (20mmol), the others are the same as in Example 1, and finally 6.09g of light yellow benzoyloxy spirofluorenoxanthyl-m-cresol group is obtained. Benzoxazine monomer, yield 70.2%.
[0027] H NMR test results (500M, CDCl 3 , ppm): 6.66~8.08 (m, 28H, Ar-H), 5.39 (s, 4H, O-CH 2 -N), 4.67 (s, 4H, Ar-CH 2 -N), 2.31 (s, 6H, Ar-CH 3 ); infrared spectrum test results (KBr, cm -1 ): 1729 (stretching vibration of carbonyl group), 1383 (swing vibration of methylene on oxazine ring), 1260 and 1068 (C-O-C asymmetric and symmetric stretching vibration), 1181 (C-N-C asymmetric stretching vibration vibration), 958 (out-of-plane bending vibration of the C—H oxazine ring, which is also a characteristic peak of the oxazine ring). Combined with H NMR and IR spectra, it was confirmed that the obtained product was the target monomer.
[0028] The curing conditions are the same as in Ex...
Embodiment 3
[0030] Except that the raw material phenol in the third step is replaced by m-methoxyphenol, and the dosage is 2.48g (20mmol), the others are the same as in Example 1, and finally 6.41g of light yellow benzoyloxy-containing spirofluorenoxanthyl-m-methoxyphenol is obtained. Oxyphenoxybenzoxazine monomer, yield 71.3%.
[0031] H NMR test results (500M, CDCl 3 , ppm): 6.61~7.92 (m, 28H, Ar-H), 5.40 (s, 4H, O-CH 2 -N), 4.62 (s, 4H, Ar-CH 2 -N), 3.83 (s, 6H, Ar-OCH 3 ); infrared spectrum test results (KBr, cm -1 ): 1727 (stretching vibration of carbonyl group), 1385 (swing vibration of methylene on oxazine ring), 1269 and 1071 (asymmetric stretching vibration of C—O—C), 1181 (asymmetric stretching vibration of C—N—C vibration), 960 (out-of-plane bending vibration of the C—H oxazine ring, which is also a characteristic peak of the oxazine ring). Combined with H NMR and IR spectra, it was confirmed that the obtained product was the target monomer.
[0032] The curing conditions...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com