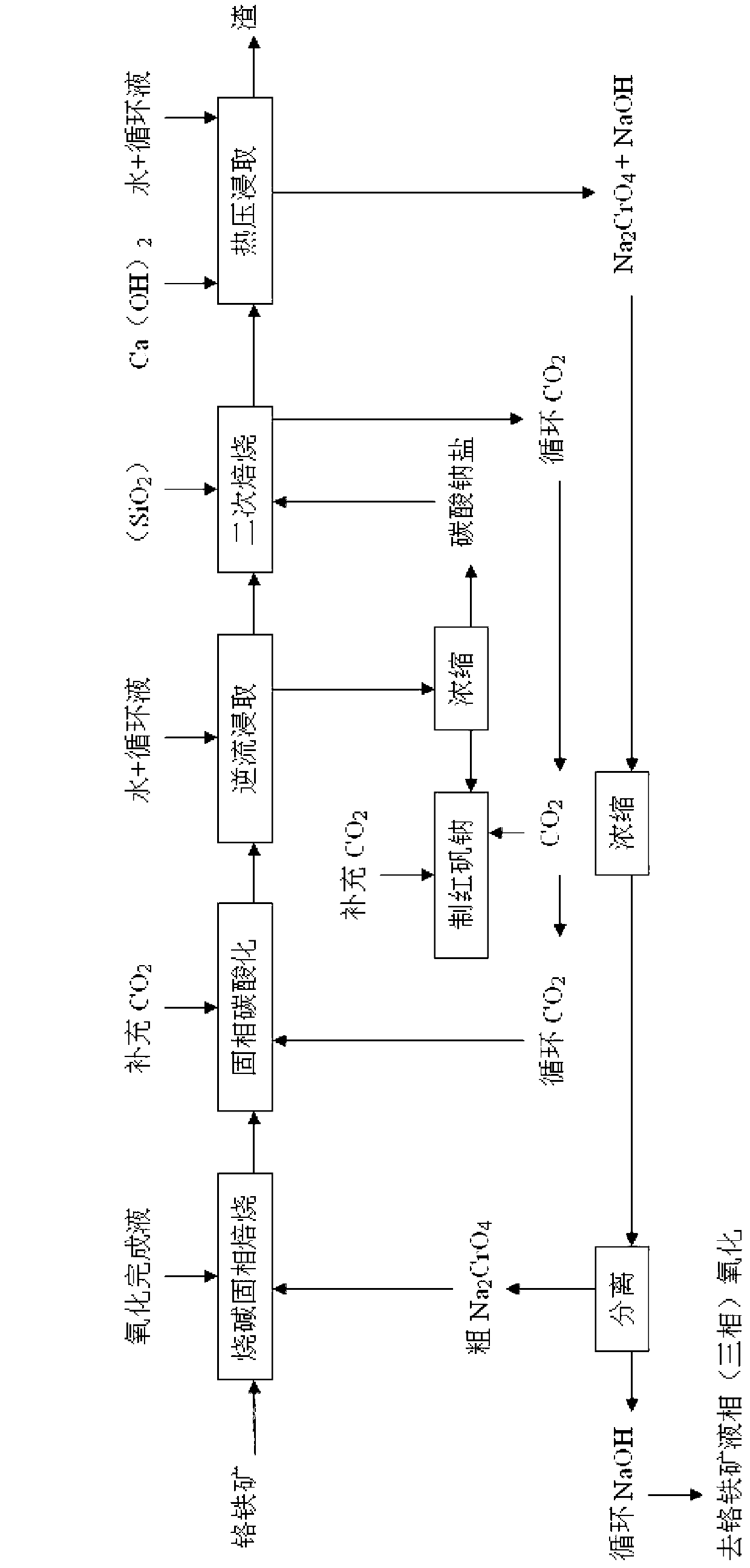
Separation method for completion liquid of caustic soda liquid-phase oxidation of chromite
A technology of chromite and caustic soda, which is applied in the field of chromate, can solve the problems of difficult solid-liquid separation and unrealized industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] with a Cr 2 o 3 38.42%, Fe 2 o 3 15.24%, MgO 17.78%, Al 2 o 3 23.24%, SiO 3 4.46%, CaO 0.91% chromite, oxidation completion solution obtained by three-phase oxidation, containing Na 2 CrO 4 33.05%, NaOH 28.15%, NaAlO 2 9.27%, Na 2 SiO 3 1.97%.
[0075] For 1 ton, 0.828 tons of the above oxidation completion liquid and 0.172 tons of crude product sodium chromate (containing Na 2 CrO 4 90.91%), calculated according to formula (7) and formula (8), the minimum amount of chromite that needs to be added is 0.73 tons, and the maximum amount is 1.0 tons.
[0076] Spray 0.828 tons of this oxidized solution on 0.85 tons of chromite and 0.172 tons of crude sodium chromate at 280°C, dry and granulate at 90—280°C, so that 90% of the particles are within 0.05—5mm within range. Calcined in air, the temperature of the material was slowly (within 1.5 hours) raised from 280°C to 680°C and held at 680°C for 50 minutes.
[0077] After cooling the material to room ...
Embodiment 2
[0083] Oxidation completion liquid and crude product sodium chromate identical with embodiment 1 composition and quantity, but use another kind of ore with higher silicon content, i.e. Cr 2 o 3 34.84%, Fe 2 o 3 18.56%, MgO 20.09%, Al 2 o 3 13.24%, SiO 3 11.66%, CaO 1.09% chromite. By 0.828 tons of the above oxidation completion liquid and 0.172 tons of crude product sodium chromate (containing Na 2 CrO 4 90.91%), calculated according to formula (7) and formula (8), the minimum amount of chromite that needs to be added is 0.81 tons, and the maximum amount is 1.10 tons.
[0084] Spray 0.828 tons of this oxidation-completed liquid on 0.9 tons of chromite and 0.172 tons of crude sodium chromate at 240°C, dry and granulate at 80—240°C, so that 90% of the particles are within 0.05—5mm within range. Roasting in the air, the temperature of the material is slowly (within 1.5 hours) raised from 240°C to 600°C for 0.5 hour, and then heated to 650°C for 0.5 hour.
[008...
Embodiment 3
[0092] with a Cr 2 o 3 46.31% of chromite, obtained through three-phase oxidation to obtain the oxidation completion solution, plus the hot-pressed leaching solution of one process cycle to concentrate the crude product sodium chromate, comprehensively calculate the Na content of the material 2 CrO 4 43.44%, NaOH 25.64%, NaAlO 2 5.91%, Na 2 SiO 3 1.70%, water 16.99%, insoluble matter 6.32%.
[0093] When dealing with this oxidation completion liquid, use a Cr-containing 2 o 3 34.84%, Fe 2 o 3 18.56%, MgO 20.09%, Al 2 o 3 13.24%, SiO 3 11.66%, CaO 1.09% chromite. By 0.842 tons of the above oxidation completion liquid and 0.158 tons of crude product sodium chromate (containing Na 2 CrO 4 90.91%), calculated according to formula (7) and formula (8), the minimum amount of chromite that needs to be added is 0.81 tons, and the maximum amount is 1.07 tons.
[0094] 0.842 tons of the above-mentioned oxidation completion solution is sprayed on 0.95 tons of th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| cycle efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com