Acidic ionic liquid catalysis method for preparing phenyl ethyl phenyl ethane capacitor insulating oil
A technology of phenylethylphenylethane and acidic ionic liquid, which can be applied to organic liquid insulators, organic insulators, hydrocarbon production from halogen-containing organic compounds, etc. The effect that the range and catalyst activity remain unchanged
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
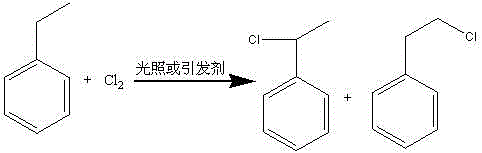
[0030] Add 5 mol of ethylbenzene into reactor A, and under stirring, 110°C and 5 g of azobisisobutyronitrile, 1 mol of chlorine gas is passed into reactor A at a constant speed for 3 hours. After the chlorine gas is passed through, continue the reaction for 2 hour, the hydrogen chloride gas generated in the reaction process is absorbed by water to obtain the discharge of by-product hydrochloric acid, and is down to room temperature, and the mixed material of α-chloroethylbenzene, β-chloroethylbenzene and ethylbenzene obtained;
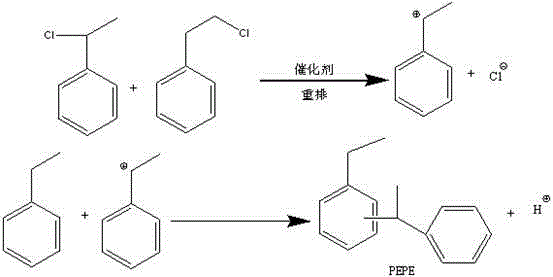
[0031] Add the mixed material in reactor A to 5mol ethylbenzene and acidic ionic liquid catalyst 12g (CH3CH2)3NHCl / XZnCl2 (X=1.1~5), acidic ionic liquid catalyst 2g (CH3)3NHCl at 60°C at one time / XZnCl2 (X=1.1~5) mixture was reacted in Reactor B; after the mixing was completed, the reaction was continued at 100°C for 6 hours, and α-chloroethylbenzene and β-chloroethylbenzene were completely detected by gas chromatography. After consumption, stop the r...
Embodiment 1-2
[0033] Add 7 mol of ethylbenzene into reactor A, under stirring, 110°C and ultraviolet light irradiation triggering, 1 mol of chlorine gas is passed into reactor A at a constant speed for 3 hours, after the chlorine gas is passed through, continue to react for 2 hours, after the reaction The hydrogen chloride gas generated in the process is absorbed by water to obtain the by-product hydrochloric acid and discharged, and kept warm to obtain a mixture of α-chloroethylbenzene, β-chloroethylbenzene and ethylbenzene;
[0034] Add the mixed material in reactor A to 5mol ethylbenzene and 3g acidic ionic liquid catalyst (CH3CH2)3NHCl / XZnCl2 (X=2~3) and 2g acidic ionic liquid catalyst (CH3)3NHCl at 100°C at one time / XZnCl2 (X=2~3) mixture was reacted in Reactor B; after the mixing was completed, the reaction was continued at 100°C for 5 hours, and α-chloroethylbenzene and β-chloroethylbenzene were detected by gas chromatography. After consumption, stop the reaction, absorb the hydroge...
Embodiment 1-3
[0036] Add 4 mol of ethylbenzene to reactor A, and under the action of stirring, 120°C and ultraviolet light irradiation, feed 1 mol of chlorine gas into reactor A at a constant speed for 3 hours. The hydrogen chloride gas generated in the process is absorbed by water to obtain the by-product hydrochloric acid to be discharged, and cooled to room temperature to obtain a mixture of α-chloroethylbenzene, β-chloroethylbenzene and ethylbenzene;
[0037] Add the mixed material in the reactor A to the reactor B containing a mixture of 10mol ethylbenzene and 5g acidic ionic liquid catalyst (CH3CH2)3NHCl / XZnCl2 (X=1.1~5) at 100°C for reaction; the mixing is completed Afterwards, the reaction was continued at 80°C for 8 hours. After the complete consumption of α-chloroethylbenzene and β-chloroethylbenzene was detected by gas chromatography, the reaction was stopped, and the hydrogen chloride gas generated during the reaction was absorbed with water to obtain the by-product hydrochloric ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com