Zinc cathode electrolyte used for redox cells
An electrolyte and zinc negative electrode technology, applied in the field of electrochemical redox batteries, can solve the problems of reducing current efficiency and hindering the industrial development of batteries
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0017] Explanation: In the examples described below, SA means methanesulfonic acid.
example 1
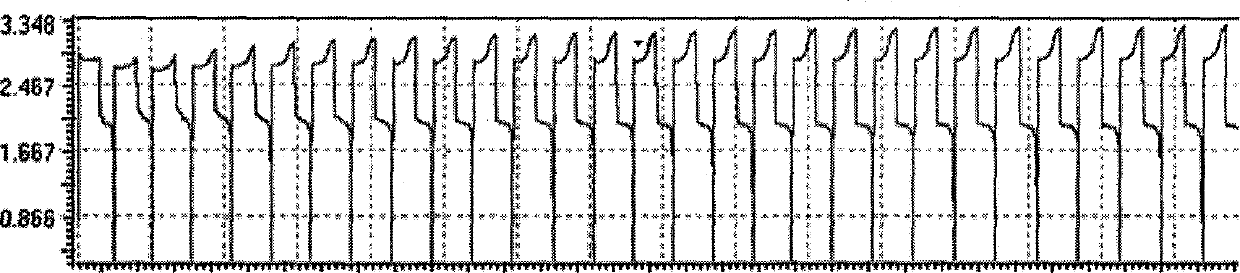
[0018] Example 1, the negative electrolyte does not contain the zinc cerium battery of additive.
[0019] (1) With 100ml of 0.8mol / L Ce 2 (CO 3 ) 3 And 100ml1.0mol / L Zn(SA) 2 .
[0020] - Weigh 61.5g methanesulfonic acid and Ce 2 (CO 3 ) 3 , formulated as 0.8mol / L Ce(SA) 3 +4.0mol / L SA catholyte.
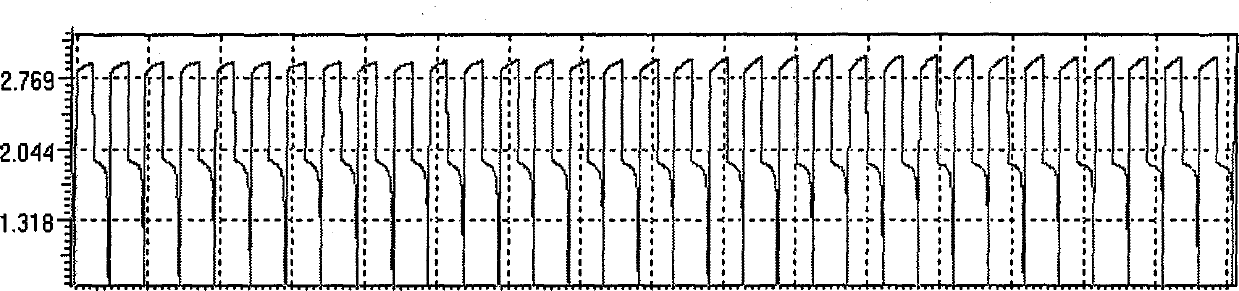
[0021] - Weigh 8.14g ZnO and 38.40g methanesulfonic acid to prepare 1.0mol / L Zn(SA) 2 . The positive and negative chambers of the battery are separated by a cation exchange membrane. The positive and negative materials are graphite felt and carbon-plastic composite electrode respectively, and the reaction area between graphite felt and electrode is 4cm 2 . The catholyte is the 0.8mol / LCe(SA) prepared in ① 3 +4.0mol / LSA, the negative electrode solution is 1.0mol / LZn(SA) prepared in ② 2 . The charging and discharging current is 120mA, and the charging time is 30min. The maximum charge voltage of the battery is 3.18V, the maximum discharge voltage is 1.92V, the discharg...
example 2
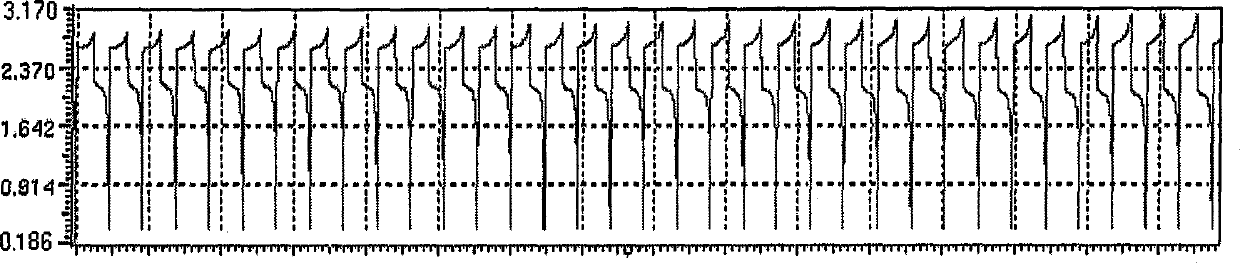
[0022] Example 2, the negative electrolyte contains the zinc cerium battery of additive.
[0023] (1) positive electrode electrolyte and negative electrode electrolyte are the same as example 1.
[0024] (2) Add the following additives to 100ml negative electrode solution:
[0025] -weigh boric acid 2.0g, borax 1.5g;
[0026] -weigh 0.02g of indium hydroxide;
[0027] -Weigh lead oxide 0.02g;
[0028] - Take 0.5 g of cetyltrimethylammonium bromide.
[0029] - Stir and dissolve the added additives under heating conditions, and cool to room temperature.
[0030] (3) Battery assembly and experimental parameter setting According to the description in Example 1, charge and discharge tests were carried out. The maximum charge voltage of the battery is 2.96V, the maximum discharge voltage is 2.22V, the discharge time above 1V within one cycle can reach 27min, the discharge time above 0.2V can reach 27.5min, and the Coulombic efficiency is 91.7%. The results show that after addi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com