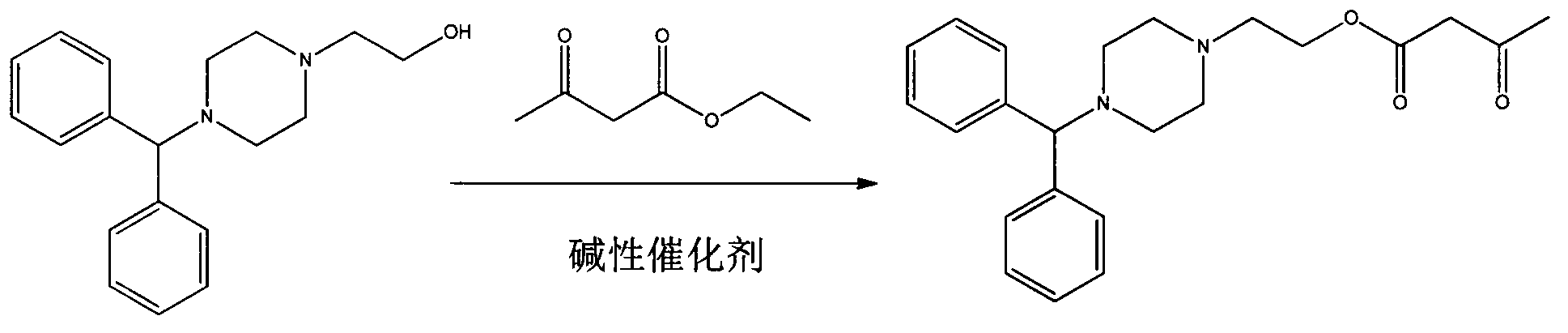
Preparation method of manidipine intermediate 2-(4-diphenylmethyl piperazinyl)ethyl acetoacetate
A technology of ethyl acetoacetate and benzhydrylpiperazine, which is applied in the field of preparation of manidipine intermediate 2-ethyl acetoacetate, achieves the effects of mild reaction, high yield and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] A kind of preparation method of manidipine intermediate 2-(4-benzhydrylpiperazine) ethyl acetoacetate, comprises the steps:
[0020] (1) Add 9.7g (0.405mol) of sodium hydride to 500mL of toluene, heat up to 90°C, add dropwise 100g (0.337mol) of 1-benzhydryl-4-(2-hydroxyethyl)piperene The toluene (300mL) solution of oxazine was stirred for about 30min, and then 52.68g (51.19ml, 0.405mol) ethyl acetoacetate was added dropwise, and reacted at this temperature for 5 hours;
[0021] (2) After the obtained reaction solution was concentrated under reduced pressure, it was extracted with ethyl acetate (500mL×3) and saturated brine, the organic phases were combined, dried, and concentrated to obtain an oil, which was dissolved in 600mL of dichloromethane , then pass hydrogen chloride gas, stir and crystallize, and filter to obtain a white solid;
[0022] (3) The obtained white solid was dissolved in 20 times the volume of water, and 30% potassium hydroxide aqueous solution was ...
Embodiment 2
[0024] A kind of preparation method of manidipine intermediate 2-(4-benzhydrylpiperazine) ethyl acetoacetate, comprises the steps:
[0025] (1) Add 72.82g (1.348mol) of sodium methylate to 800mL of dichloromethane, heat up to 40°C, and add 200g (0.674mol) of 1-benzhydryl-4-(2-hydroxyethyl) dropwise. ) dichloromethane (400mL) solution of piperazine, after stirring for about 30min, add dropwise 52.68g (102.38ml, 0809mol) ethyl acetoacetate, react at this temperature for 8 hours;
[0026] (2) After the obtained reaction solution was concentrated under reduced pressure, it was extracted with ethyl acetate (800mL×3) and saturated brine, the organic phases were combined, dried, and concentrated to obtain an oil, which was dissolved in 600mL of dichloromethane , then pass hydrogen chloride gas, stir and crystallize, and filter to obtain a white solid;
[0027] (3) Dissolve the gained white solid in 20 times the volume of water, add dropwise 30% potassium hydroxide aqueous solution t...
Embodiment 3
[0029] (1) Add 121.10g (0.876mol) of potassium carbonate to 800mL of toluene, heat up to 90°C, and dropwise add 130g (0.438mol) of 1-benzhydryl-4-(2-hydroxyethyl)piperene The toluene (300mL) solution of oxazine was stirred for about 30min, and then 79.02g (66.55ml, 0.526mol) ethyl acetoacetate was added dropwise, and reacted at this temperature for 6 hours;
[0030] (2) After the obtained reaction solution was concentrated under reduced pressure, it was extracted with ethyl acetate (500mL×3) and saturated brine, the organic phases were combined, dried, and concentrated to obtain an oil, which was dissolved in 600mL of dichloromethane , then pass hydrogen chloride gas, stir and crystallize, and filter to obtain a white solid;
[0031] (3) Dissolve the obtained white solid in 20 times the volume of water, add 30% potassium hydroxide aqueous solution dropwise under stirring conditions until the pH is 10-11, place it at 4°C for a period of time, suction filter, and dry to obtain 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com