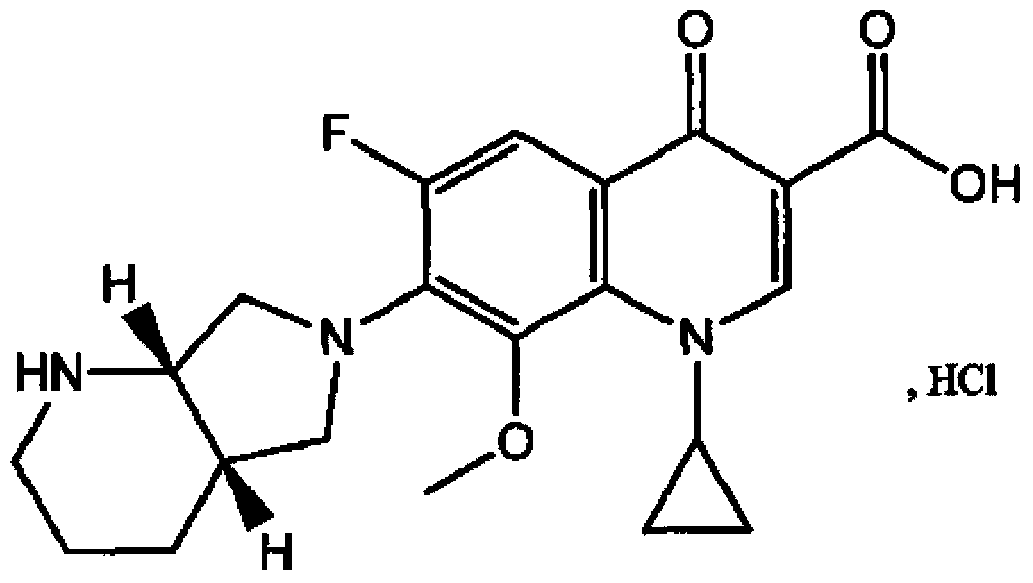
Moxifloxacin hydrochloride injection aqua
A technology of moxifloxacin hydrochloride and water preparation, which is applied in the field of pharmaceutical preparations, can solve the problem that the concentration should not be higher than 3.0%, and achieve the effect of improving storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The specific operation steps of the above-mentioned preparation method are to adjust the solution of the drug and the pH value at an operating temperature of 30-50°C. During the adjustment, the pH value at the end point of the adjustment is determined according to the pH value drop curve and the target pH value and the drop range. After the end point, the heating was stopped, and the mixture was stirred weakly or allowed to stand for 2-3 hours at room temperature. Then continue the follow-up operation. It is easy to understand that the standing time can also be other times, such as less than 2 hours or more than 3 hours, which can be regarded as equivalent replacements.
[0025] As a further preference, the preparation obtained by the above operations is encapsulated in a carbon dioxide atmosphere. The preparation obtained by the method is still stable after being stored for two years at a temperature of 15° C., no precipitation occurs, and the effective content is abo...
Embodiment 1
[0028] Take 4.0g of moxifloxacin hydrochloride raw material, add 80ml of water for injection, stir and dissolve below 40°C, slowly add Na 2 CO 3 Solution until the drug is completely dissolved, then maintain the temperature, continue to drop Na 2 CO 3 solution to pH 7.3, stop heating, add Na 2 CO 3 The solution was about 15ml, and the pH value dropped to 7.1 under weak stirring at room temperature for 3 hours. Filtered at 0.45 μm and 0.22 μm, sampled for measurement, supplemented with water for injection to the full amount, and finally packaged. The preparation has no crystal precipitation when stored at 1-5°C for 12 months, and the effective content is not less than 90% when stored at 15-25°C for 12 months.
Embodiment 2
[0030] Take 6.0g of moxifloxacin hydrochloride raw material, add 50ml of water for injection, stir and dissolve below 40°C, slowly add Na 2 CO 3 Solution until the drug is completely dissolved, then maintain the temperature, continue to drop Na 2 CO 3 solution to pH 7.8, stop heating, add Na 2 CO 3 The solution was about 45ml, and the pH value dropped to 7.4 under weak stirring at room temperature for 3 hours. Filtered at 0.45 μm and 0.22 μm, sampled for measurement, supplemented with water for injection to the full amount, and finally packaged. The preparation has no crystal precipitation when stored at 1-5°C for 12 months, and the effective content is not less than 90% when stored at 15-25°C for 12 months.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com