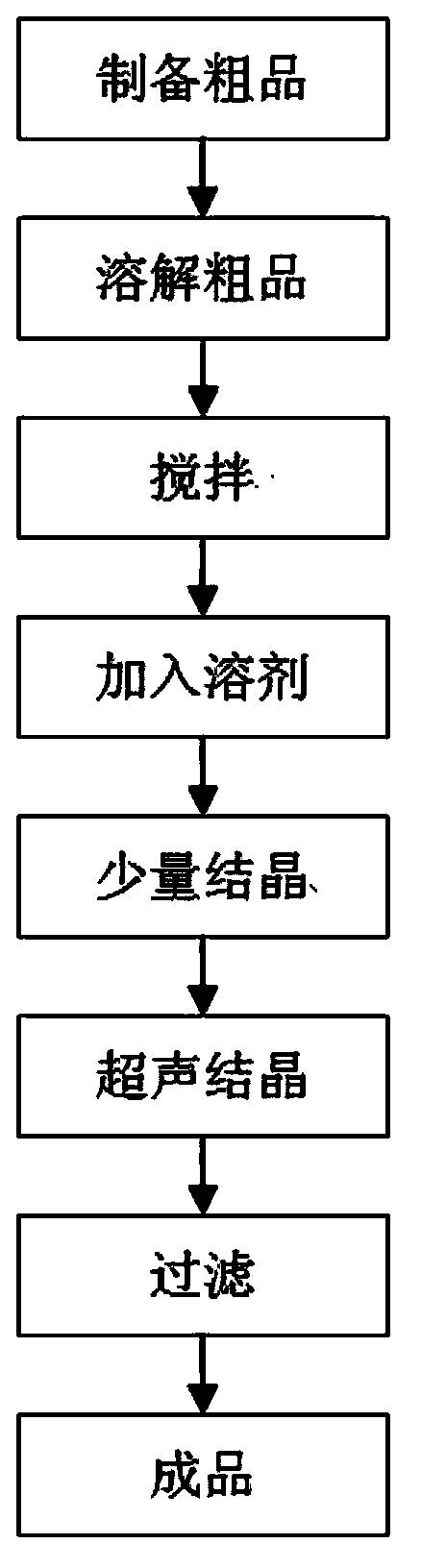
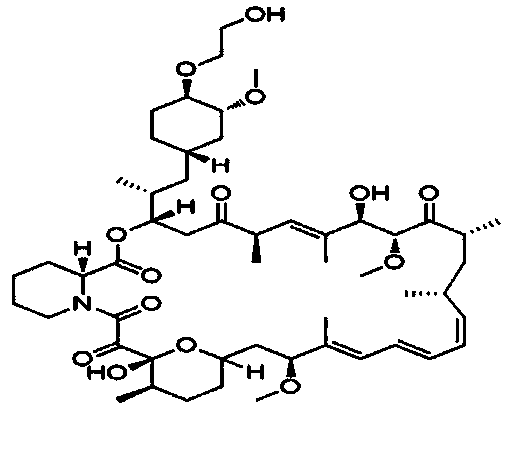
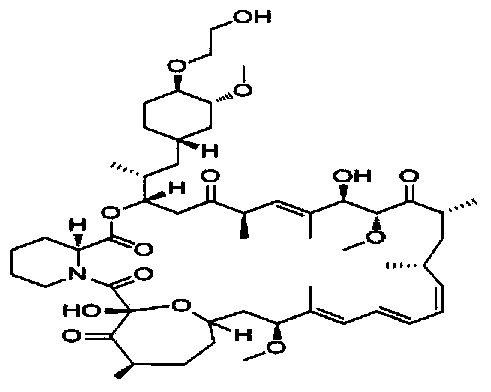
Everolimus crystallization purification method
A technology for everolimus and a purification method, which is applied in the field of everolimus crystallization and purification, can solve problems such as difficulty in purification of everolimus, and achieve the effects of good crystallization effect, removal or reduction of other impurities, and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] 20 g of the synthesized everolimus raw material was subjected to normal phase column chromatography. The normal-phase silica gel is ordinary normal-phase silica gel, the particle size is 200-300 mesh, and the dosage of silica gel is 200 g; the chromatography resolver is: ethyl acetate:petroleum ether=7:3. Collected in sections to obtain 10 g of crude everolimus (the content of everolimus is 95.51%, the content of isomers is 3.35%, and the content is determined by the area normalization method).
[0060] Take 5 g of the above-mentioned crude everolimus, dissolve the crude everolimus in 50 ml of methanol containing 5‰ V / V of water, wherein the pH value of the water is 3.0, adjust with glacial acetic acid, and stir for 2 hours at 5°C; Add 250ml of n-heptane to the above solution, continue to stir until a small amount of crystals are precipitated, then use ultrasonic crystallization until a large amount of crystals are precipitated, and filter to obtain 4.62g of everolimus ...
Embodiment 2
[0062] Take 5 g of the crude everolimus product described in Example 1, dissolve the crude product of everolimus with 50 ml of ethanol containing 6‰ V / V of water, wherein the pH value of the water is 3.0, adjust with glacial acetic acid, and stir at 7°C for 2.5 h; add 300ml of n-heptane to the above solution, continue to stir until a small amount of crystals form, then use ultrasonic crystallization until a large amount of crystals are precipitated, filter to obtain 4.65g of everolimus finished product (wherein, the content of everolimus is 98.98%, the content of isomers is 0.39%, and the individual content of other impurities is less than 0.2%, and the content is determined by the area normalization method).
Embodiment 3
[0064] 220 g of the synthesized everolimus raw material was subjected to normal phase column chromatography. The normal-phase silica gel is ordinary normal-phase silica gel, the particle size is 200-300 mesh, and the amount of silica gel is 2.2 kg; the chromatographic analytical solvent is: ethyl acetate:petroleum ether=7:3. Collected in sections to obtain 105 g of crude everolimus (wherein, the content of everolimus is 95.11%, and the content of isomers is 4.87%, and the content is determined by the area normalization method).
[0065] Take 50 g of the crude everolimus above, and dissolve the crude everolimus with 500 ml of isopropanol containing 7‰ V / V of water, wherein the pH of the water is 3.0, adjust with glacial acetic acid, and stir for 3 hours at 8°C; Then add 4000ml of n-hexane to the above solution, continue to stir until a small amount of crystals form, then use ultrasonic crystallization until a large amount of crystals are precipitated, filter to obtain 47.45g of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com