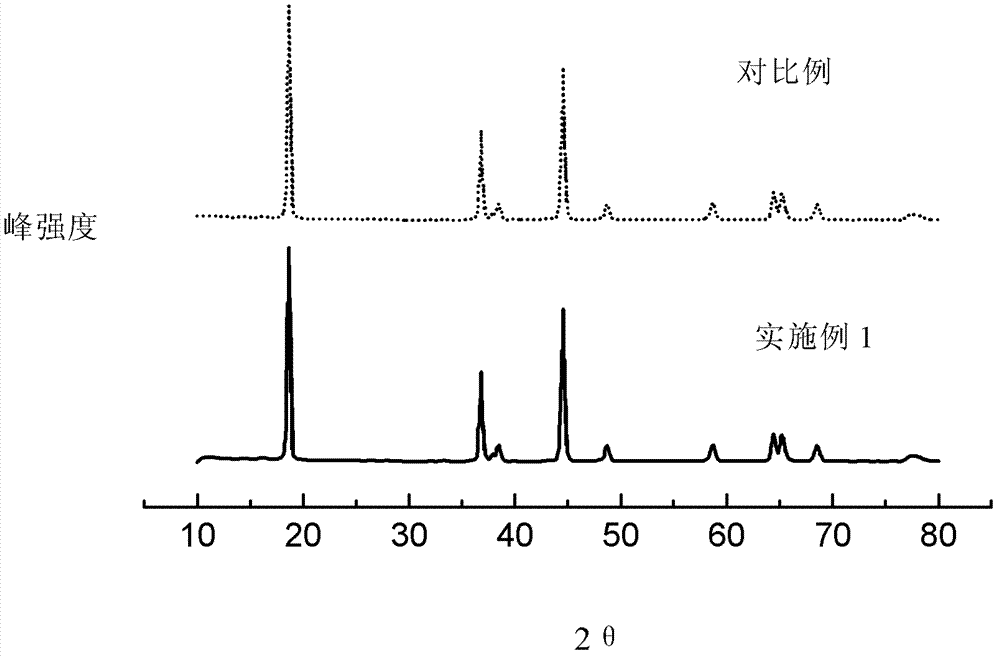
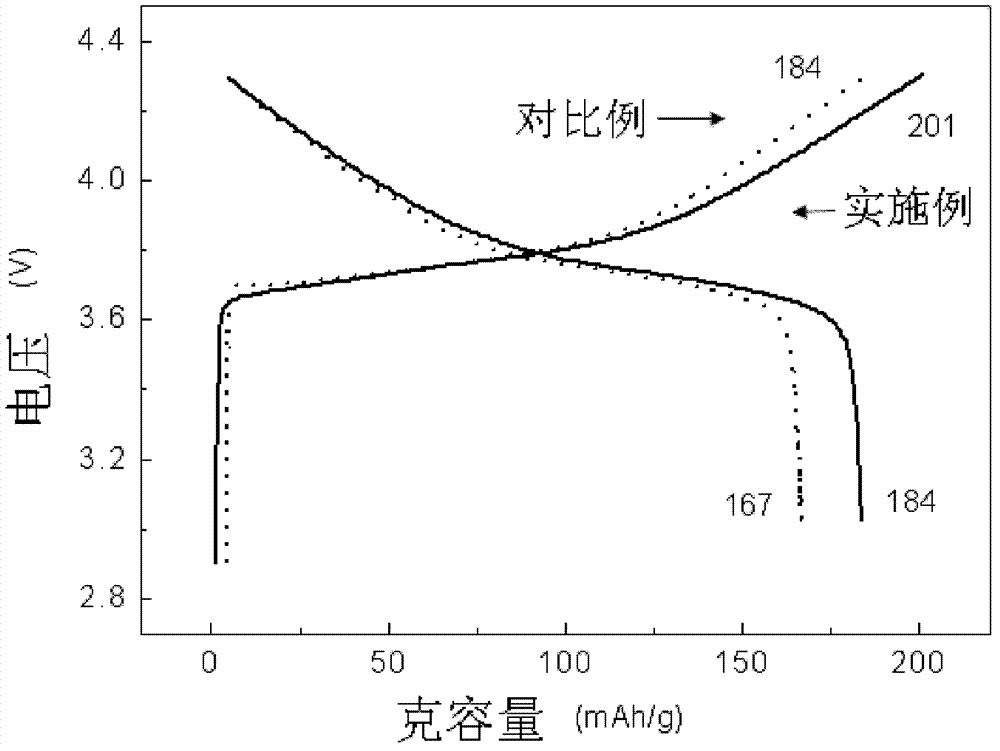
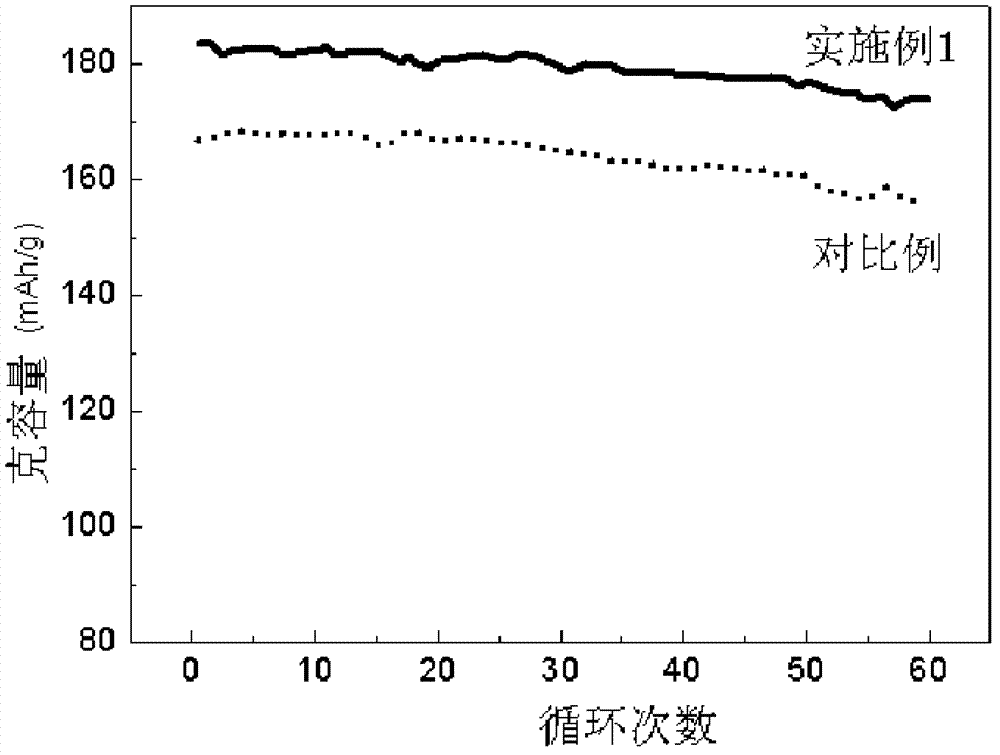
Gradient distribution multivariate composite material precursor as well as preparation method and application thereof
A technology of gradient distribution and precursors, applied in electrical components, battery electrodes, circuits, etc., can solve the problems of discharge specific capacity, stability, rate and safety performance to be improved, so as to improve electrical conductivity and battery rate performance, The effect of high specific capacity and high discharge specific capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] First prepare the salt solution A of the material core: nickel sulfate, cobalt sulfate, manganese sulfate, magnesium sulfate in molar ratio (78%: 10%: 10%: 2%) to prepare a salt solution with a concentration of 2mol / L. Other salt solution B, C, D, E are respectively by nickel sulfate, cobalt sulfate, manganese sulfate, magnesium sulfate molar ratio: B (58%: 20%: 20%: 2%); C (50%: 25%: 25%); D (38%: 30%: 30%: 2%); E (32.7%: 32.7%: 32.7%: 2%), the nickel content of the salt solution gradually decreased.
[0037] The salt solution A was injected into the reactor at a speed of 0.4 L / h through a metering pump with a rotation speed of 400 rps, and the temperature of the reactor was kept at 50°C. At the same time, inject the NaOH solution with a mass percentage concentration of 15-25% ammonia water and 8-12mol / L, pay attention to adjust the flow rate of the alkali solution, and keep the pH value between 9-12 through the online pH tester; after the salt solution A is completely...
Embodiment 2
[0046] As shown in Table 1, first prepare the salt solution A of the material core: nickel sulfate, cobalt sulfate, manganese sulfate, and zinc sulfate are prepared in molar ratios (69%: 15%: 15%: 1%) with a concentration of 2mol / L. saline solution. Other salt solution B, C, D, E are respectively by nickel sulfate, cobalt sulfate, manganese sulfate, zinc sulfate molar ratio: B (59%: 20%: 20%: 1%); C (49%: 25%: 25%: 1%); D (39%: 30%: 30%: 1%); E (33%: 33%: 33%: 1%). The nickel content of the brine gradually decreases.
[0047] The preparation process was the same as in Example 1. The tap density, initial discharge capacity, capacity retention rate of 60 cycles at room temperature, and capacity retention rate after 20 cycles at a high temperature of 50°C are shown in Table 2.
Embodiment 3
[0049] As shown in Table 1, first prepare the salt solution A of the core of the material: nickel sulfate, cobalt sulfate, manganese sulfate, aluminum sulfate are prepared in molar ratio (67%: 15%: 15%: 3%) with a concentration of 2mol / L saline solution. Other salt solution B, C, D, E are respectively by nickel sulfate, cobalt sulfate, manganese sulfate, aluminum sulfate molar ratio: B (57%: 20%: 20%: 3%); C (47%: 25%: 25%: 3%); D (37%: 30%: 30%: 3%); E (32.3%: 32.3%: 32.3%: 3%). The nickel content of the brine gradually decreases.
[0050] The preparation process was the same as in Example 1. The tap density, initial discharge capacity, capacity retention rate of 60 cycles at room temperature, and capacity retention rate after 20 cycles at a high temperature of 50°C are shown in Table 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com