Method for collection of ruthenium or ruthenium compound
A ruthenium compound and recovery method technology, applied in silicon compounds, chemical instruments and methods, separation methods, etc., can solve the problems of limited use of materials, complicated procedures, strong corrosiveness, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
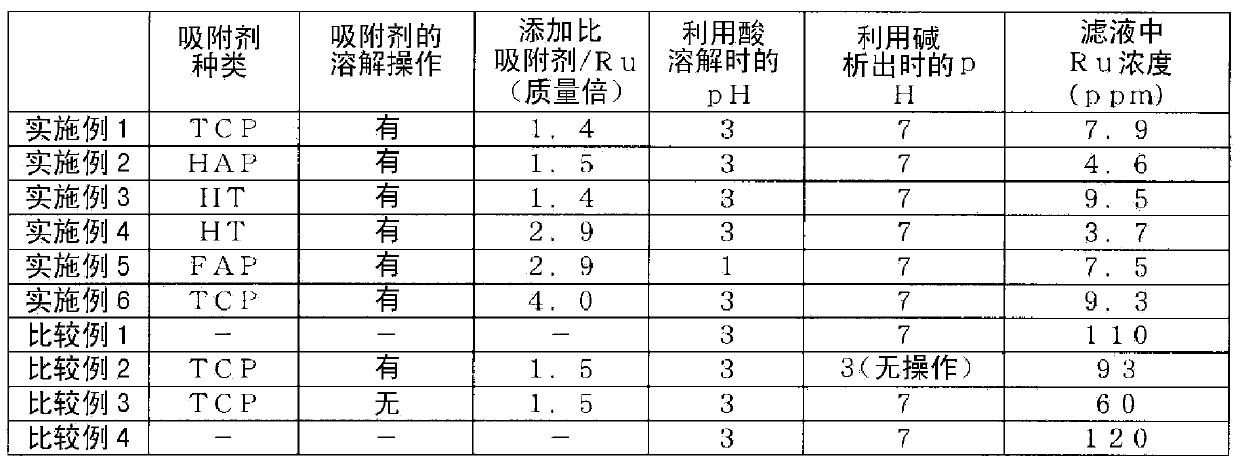
[0079] In a 500ml conical beaker made of glass, 340g of an aqueous solution containing 680ppm ruthenium, 8wt% sodium chloride and 0.5wt% acetic acid, and 1.4 mass times of ruthenium, i.e. 0.32g, was added tricalcium phosphate as an inorganic adsorbent (Wako Pure Chemical Industries, Ltd.) Tricalcium phosphate manufactured by the company. Hereinafter referred to as "TCP"), start stirring with a magnetic stirrer. Next, in order to dissolve the inorganic adsorbent, a 70wt% sulfuric acid solution was added using a safety pipette (Komagome pipette) until the pH reached 3, and stirring was continued for 30 minutes to dissolve the tricalcium phosphate. The addition amount of the sulfuric acid solution at this time was 7.5 g. Next, in order to precipitate the dissolved inorganic adsorbent, a 25wt% sodium hydroxide solution was added using a safety pipette until the pH reached 7, and then stirring was continued for 30 minutes to precipitate tricalcium phosphate. The addition amount of ...
Embodiment 2
[0081] In addition to using 290 g of an aqueous solution containing 690 ppm of ruthenium, and 0.29 g of hydroxyapatite, which is 1.5 times the mass of ruthenium, as an inorganic adsorbent (APATITE HAP manufactured by Wako Pure Chemical Industries, Ltd., monoclinic crystal, abbreviated as "HAP") , Implement the same operation as in Example 1. The concentration of ruthenium in the obtained filtrate was 4.6 ppm.
Embodiment 3
[0083] In addition to using 300 g of an adsorption raw material aqueous solution containing 650 ppm of ruthenium and 0.29 g of hydrotalcite as an inorganic adsorbent (hydrotalcite manufactured by Wako Pure Chemical Industries, Ltd., hereinafter referred to as "HT") that is 1.4 times the mass of ruthenium, and The same operation as in Example 1. The concentration of ruthenium in the obtained filtrate was 9.5 ppm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| rate of recovery | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com